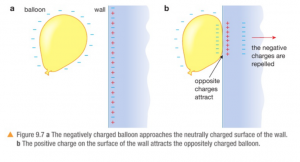
The empirical formula shows the simplest whole-number ratio between atoms/ions in a compound.
The molecular formula shows the actual number of atoms of each type of element in a molecule.
For example, for ethane:
Molecular formula = C2H6
Empirical formula = CH3
Here are some more examples:
| Name | Molecular formula | Empirical Formula |
|---|---|---|
| pentene | C5H10 | CH2 |
| butene | C4H8 | CH2 |
| glucose | C6H12O6 | CH2O |
| hydrogen peroxide | H2O2 | HO |
| propane | C3H8 | C3H8 |
Notice from the table that several different molecules can have the same empirical formula, which means that it is not possible to deduce the molecular formula from the empirical formula without some additional information. Also notice that sometimes it is not possible to simplify a molecular formula into simpler whole-number ratio, in which case the empirical formula is equal to the molecular formula.