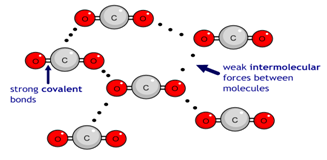
Carbon dioxide (CO2) has a simple molecular structure. This just means that it is made up of molecules.
Within each molecule are atoms bonded to each other covalently. These covalent bonds inside the molecules are strong.
However, between the molecules are weak forces of attraction that require little energy to break. These forces are not covalent bonds. This is why simple molecular substances such as carbon dioxide have a low boiling point.
So when carbon dioxide changes from a solid to a gas, for example, that process can be represented as:
CO₂ (s) → CO₂ (g)
Notice that even though there has been a dramatic change of state from solid to gas, the substance before and after the change is always made up of carbon dioxide molecules. During the change of the state the covalent bonds within each molecule remain unbroken. Instead it is the weak forces of attraction between the molecules which have been overcome.