The molecular formula shows the actual number of atoms of each element in a molecule.
The empirical formula shows the simplest whole number ratio of atoms present in a compound. So the molecular formula is a multiple of the empirical formula.
The general formula shows the relationship between the number of atoms of one element to another within a molecule. Members of a homologous series share the same general formula. The general formula for alkanes is CnH2n+2 and the general formula for alkenes is CnH2n.
A structural formula shows how the atoms in a molecule are joined together.
The displayed formula is a full structural formula which shows all the bonds in a molecule as individual lines.
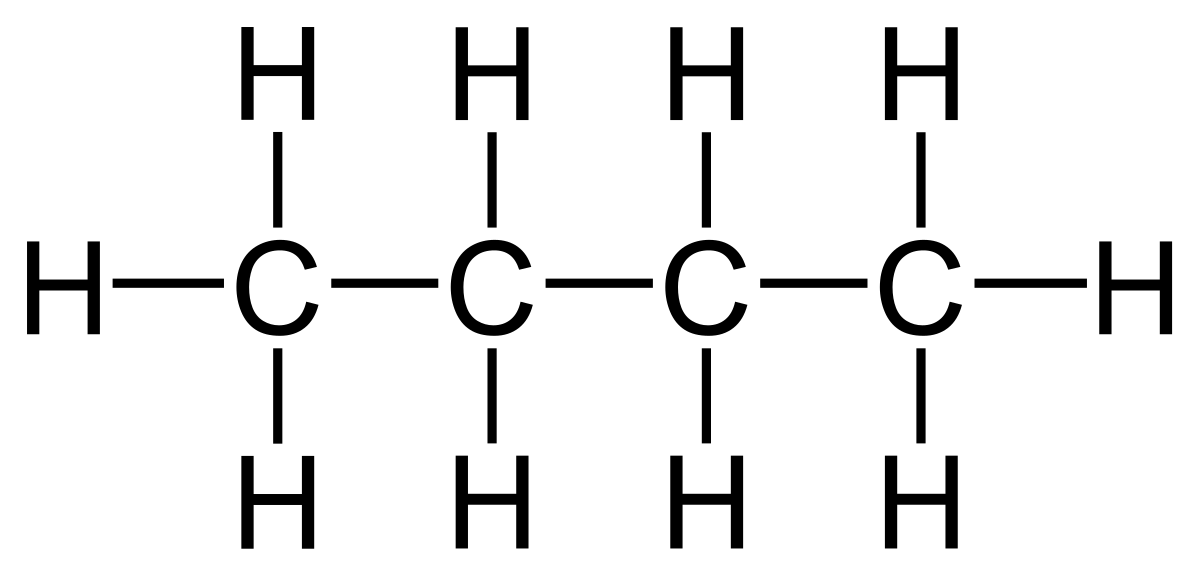
The terms above are demonstrated with the example of butane.
- Displayed formula:
- Molecular formula: C₄H₁₀
- Empirical formula: C₂H₅
- General formula (alkanes): CnH2n+2
- Structural formula: CH₃ – CH₂ – CH₂ – CH₃
The terms above are demonstrated with the example of ethene, which contains a double bond.
- Displayed formula:
- Molecular formula: C₂H₄
- Empirical formula: CH₂
- General formula (alkenes): CnH2n
- Structural formula: CH₂ = CH₂