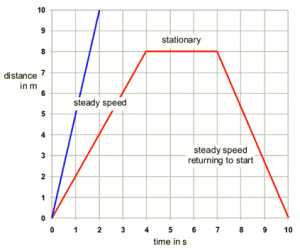
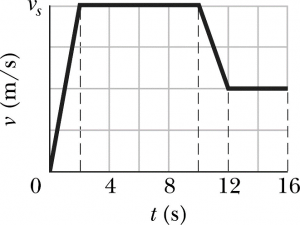
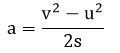- As you heat the gas, the kinetic energy of the particles increases, and thus so does their average speed.
- This means more collisions per second with the walls, and they exert a larger force on the wall.
- This causes in the total pressure being exerted by the particles to rise.
- If temperature is constant, the average speed of the particles is constant.
- If the same number of particles is placed in a container of smaller volume they will hit the walls of the container more often.
- More collisions per second means that the particles are exerting a larger force on the wall over the same time, so average force exerted on the walls has increased.