P1V1 = P2V2
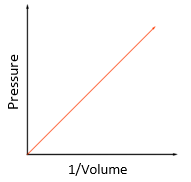
Boyle’s law:
For a fixed mass of gas at constant temperature, the pressure is inversely proportional to the volume.


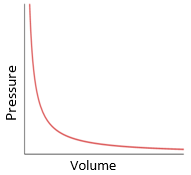
P1V1 = P2V2
Boyle’s law:
For a fixed mass of gas at constant temperature, the pressure is inversely proportional to the volume.