|
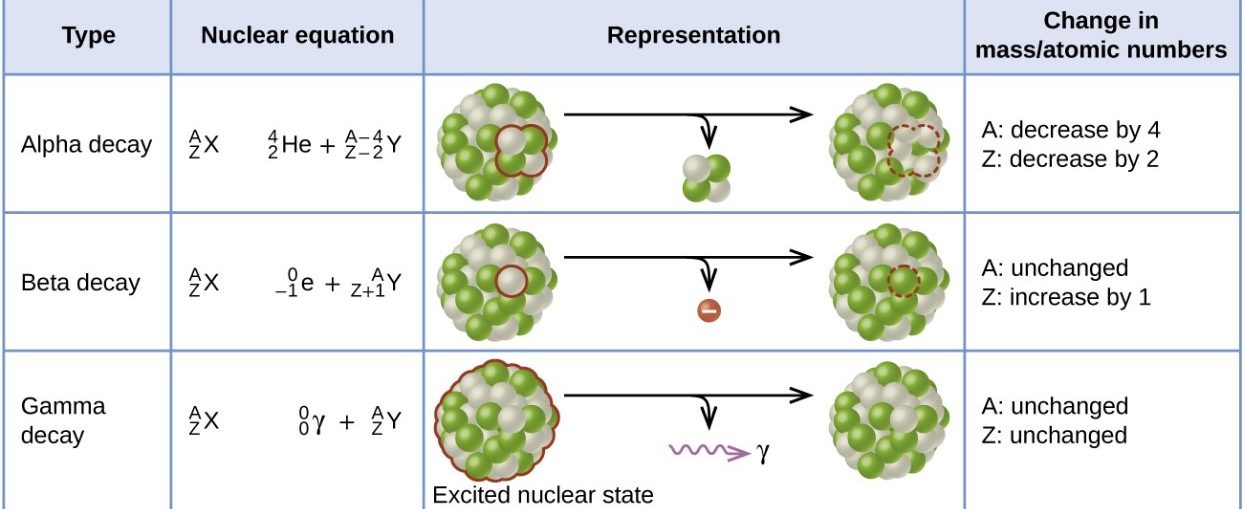
Alpha decay: · 2 protons and 2 neutrons are lost. · Mass number decreases by 4 · Atomic number decreases by 2 Beta decay · 1 neutron is converted to an electron (lost from the atom) and proton · Mass number is unchanged · Atomic number increases by 1 Gamma decay · Energy is lost from an atom in the form of an electromagnetic wave · Mass number is unchanged · Atomic number is unchanged |