Electrolysis – Tyler de Witt video
Although quite long, this Tyler de Witt video is a good summary of Electrolysis.
https://sp.outletdastintas.com.br/
https://pkmmuka.cianjurkab.go.id/
https://collegiogeometri.mb.it/
https://personal.cofadena.gob.bo/
Although quite long, this Tyler de Witt video is a good summary of Electrolysis.
Oxidation: the loss of electrons or the gain of oxygen
Reduction: the gain of electrons or the loss of oxygen
Example: The electrolysis of lead (II) bromide, PbBr2
At the cathode (negative electrode): Pb2+ (l) + 2e– → Pb (l) (reduction)
At the anode (positive electrode): 2Br– (l) → Br2 (g) + 2e– (oxidation)
Example: The electrolysis of aluminium oxide, Al2O3
At the cathode: Al3+ + 3e– → Al (reduction)
At the anode: 2O2- → O2 + 4e– (oxidation)
Example: The electrolysis of sodium chloride solution (NaCl (aq))
At the cathode: 2H+ (aq) + 2e– → H2 (g) (reduction)
At the anode: 2Cl– (aq) → Cl2 (g) + 2e– (oxidation)
Example: The electrolysis of copper sulfate solution (CuSO4 (aq))
At the cathode: Cu2+ (aq) + 2e– → Cu (s) (reduction)
At the anode: 4OH– (aq) → O2 (g) + 2H2O (l) + 4e– (oxidation)
The diagram shows an electrolytic cell.
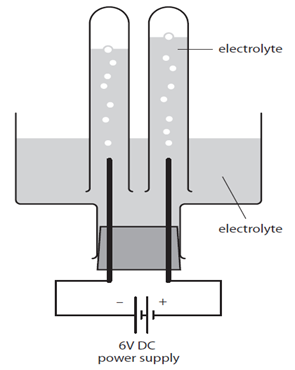
The electrolyte is an aqueous solution. For example it might be concentrated sodium chloride, NaCl (aq).
The test tubes over the electrodes must not completely cover them to make sure the ions are free to move throughout the solution.
In the case of NaCl (aq) bubbles of gas will be seen forming at the electrodes. These float up and collect in the test tubes when each gas can be tested to assess its identity.
Group 1 metals such as potassium, sodium and lithium, react with water to produce a metal hydroxide and hydrogen. For example:
lithium + water → lithium hydroxide + hydrogen
2Li (s) + 2H₂O (l) → 2LiOH (aq) + H₂ (g)
The observations for the reaction of water with either potassium or sodium or lithium have the following similarities:
In each case a metal hydroxide solution is produced.
These similarities in the reactions provide evidence that the 3 metals are in the same group of the Periodic Table (i.e. have the same number of electrons in their outer shell).
Lithium is the first element in group 1 of the Periodic Table. The observations for the reaction of lithium and water are:
Sodium is the second alkali metal in the group. The reaction of sodium and water is more vigorous than lithium’s:
Potassium is the third alkali metal in the group. The reaction of potassium and water is more vigorous than sodium’s:
When the group 1 metals react with air they oxidise, showing a similar trend in reactivity as we go down the group of the Periodic Table.
Therefore, as we go down group 1 (increasing atomic number), the elements become more reactive: Li<Na<K<Rb<Cs<Fr
From the data in the table, it is possible to deduce the properties of francium from the trends in the other group 1 metals.
For example, we can predict that francium will have a melting point around 20⁰C and a density of just over 2g/cm³.
We can also predict that francium will react violently with water, producing francium hydroxide and hydrogen.
| Alkali metal | Melting point (⁰C) | Density (g/cm³) | Reaction with water | Products |
|---|---|---|---|---|
| lithium (Li) | 181 | 0.53 | fizzing | lithium hydroxide + hydrogen |
| sodium (Na) | 98 | 0.97 | rapid fizzing | sodium hydroxide + hydrogen |
| potassium (K) | 63 | 0.86 | vigorous fizzing and lilac flame | potassium hydroxide + hydrogen |
| rubidium (Rb) | 39 | 1.53 | ? | rubidium hydroxide + hydrogen |
| caesium(Cs) | 29 | 1.88 | ? | caesium hydroxide + hydrogen |
| francium (Fr) | ? | ? | ? | ? |
The awesome Professor Sir Martyn Poliakoff shows us the reaction of some alkali metals and water.
As you go down the group the outer electron lost from the group 1 metal is further from the nucleus therefore the electron is less attracted by the nucleus and therefore more easily lost.
| Element | Colour | State at room temp |
|---|---|---|
| Chlorine (Cl2) | Green | Gas |
| Bromine (Br2) | Red-brown | Liquid |
| Iodine (l2) | Grey | Solid |
Chlorine is a toxic gas, so should be handled in a fume cupboard.
If you look at the trends in the physical properties of the halogens, Cl2, Br2, I2 you can make predictions about the properties of the other halogens.
| Element | Colour | State at room temp |
|---|---|---|
| Fluorine (F2) | Yellow | Gas |
| Astatine (At2) | Black | Solid |
Here is a great video on Fluorine, which is the most reactive halogen (group 7):
Group 7 elements are called the Halogens. As you go up group 7 (decreasing atomic number), the elements become more reactive. For example, fluorine is the most reactive and astatine is the least reactive.
A more reactive halogen will displace a less reactive halogen, e.g. chlorine will displace bromine:

By reacting a halogen solution with a potassium halide solution and making observations, the order of their reactivity can be deduced:
| Potassium chloride, KCl(aq) | Potassium bromide, KBr(aq) | Potassium iodide, KI(aq) | |
|---|---|---|---|
| Chlorine, Cl2(aq) | No change | Colourless to orange | Colourless to brown |
| Bromine, Br2(aq) | No change | No change | Colourless to brown |
| Iodine, I2(aq) | No change | No change | No change |
From the above results, chlorine displaces both bromine and iodine, and bromine displaces iodine. Therefore the order of reactivity is: chlorine is more reactive than bromine, which in turn is more reactive than iodine.
The reactions of various halogens with iron wool is a great way to see how reactive one halogen is compared to another:
Special thanks to Charlie R for suggesting I add a video on halogen displacement.
The higher up we go in group 7 (halogens) of the periodic table, the more reactive the element. The explanation concerns how readily these elements form ions, by attracting a passing electron to fill the outer shell.
In fluorine the outer electron shell is very close to the positively charged nucleus, so the attraction between this nucleus and the negatively charged electrons is very strong. This means fluorine is very reactive indeed.
However, for iodine the outer electron shell is much further from the nucleus so the attraction is weaker. This means iodine is less reactive.
Air is a mixture of different gases.
The abundance of gases in the air is as follows:
| Gas | % by volume |
|---|---|
| Nitrogen, N2 | 78.1 |
| Oxygen, O2 | 21.0 |
| Argon, Ar | 0.9 |
| Carbon dioxide, CO2 | 0.04 |
The following 3 experiments can be used to determine that oxygen (O2) makes up approximately 20% by volume of the composition of air.
Copper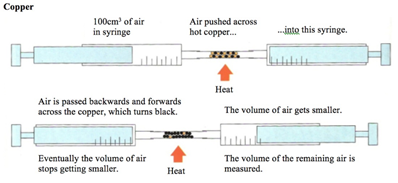
The copper is in excess and uses up the oxygen to form copper oxide (CuO).
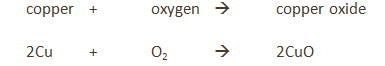
All the oxygen in the air is therefore used up, and so the volume of the air decreases by about 20% (the percentage of oxygen in air).
Iron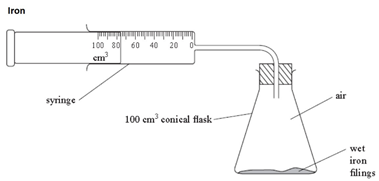
The iron reacts with the oxygen in the air (rusting).
As long as the iron and water are in excess, the total volume of air enclosed by the apparatus decreases by about a fifth (20%) over several days.
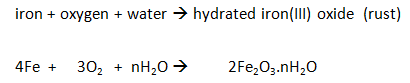
Phosphorus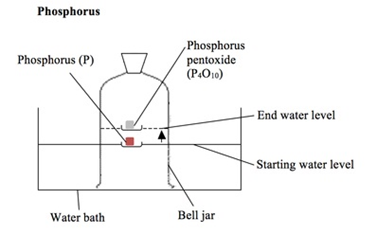
The phosphorus is lit with a hot wire.
It reacts with the oxygen in the air and causes the water level in the bell jar to rise by about 20%.
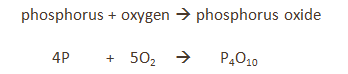
Magnesium reacts with oxygen producing a bright white flame leaving behind a white ash of magnesium oxide.
magnesium + oxygen → magnesium oxide
2Mg (s) + O₂ (g) → 2MgO
MgO is a base, which can react with an acid to give a salt and water.
Hydrogen reacts with oxygen in an explosive reaction. This is the basis of the ‘squeak pop’ test for hydrogen in test tube. With larger quantities of hydrogen this explosion can be dangerous.
hydrogen + oxygen → water
2H₂ (g) + O₂ (g) → 2H₂O (l)
Sulfur reacts with oxygen producing a blue flame.
sulfur + oxygen → sulfur dioxide
S (s) + O₂ (g) → SO₂ (g)
When sulfur dioxide (SO₂) dissolves in water it forms an acidic solution of sulfurous acid:
SO₂ (g) + H₂O (l) → H₂SO₃ (aq)
This video shows the reaction with oxygen of various elements:
thermal decomposition is the process of breaking down by heating.
On heating metal carbonates thermal decompose into metal oxides and carbon dioxide.

Observation: green powder (CuCO3) changes to a black powder (CuO)
Carbon dioxide (CO2) is a greenhouse gas.
It absorbs infra-red radiation and therefore warms the atmosphere. This leads to global warming.
This may cause climate change.
The following 3 experiments can be used to determine that oxygen (O2) makes up approximately 20% by volume of air.
Copper
The copper is in excess and uses up the oxygen to form copper oxide (CuO).
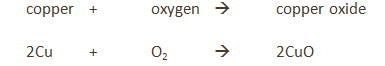
All the oxygen in the air is therefore used up, and so the volume of the air decreases by about 20% (the percentage of oxygen in air).
Iron
The iron reacts with the oxygen in the air (rusting).
As long as the iron, oxygen and water are all in excess, the total volume of air enclosed by the apparatus decreases by about a fifth (20%) over several days.
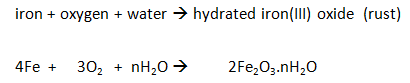
Phosphorus
The phosphorus is lit with a hot wire.
It reacts with the oxygen in the air and causes the water level in the bell jar to rise by about 20%.
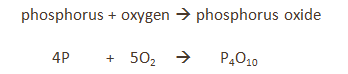
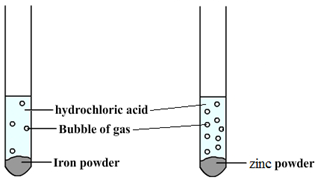 Some metals are more reactive than others.
Some metals are more reactive than others.
The order of reactivity can be determined by adding acid to different metals and observing the rate of reaction.
For example, when hydrochloric acid is added to iron (Fe) then bubbles of hydrogen are produced slowly. However, if the same acid is added to zinc (Zn) then bubbles will be produced more quickly. This tells us that zinc is more reactive than iron.
Instead of using acid, water can be used to test the relative reactivity of metals. However, many metals are too low in the reactivity series to react with water
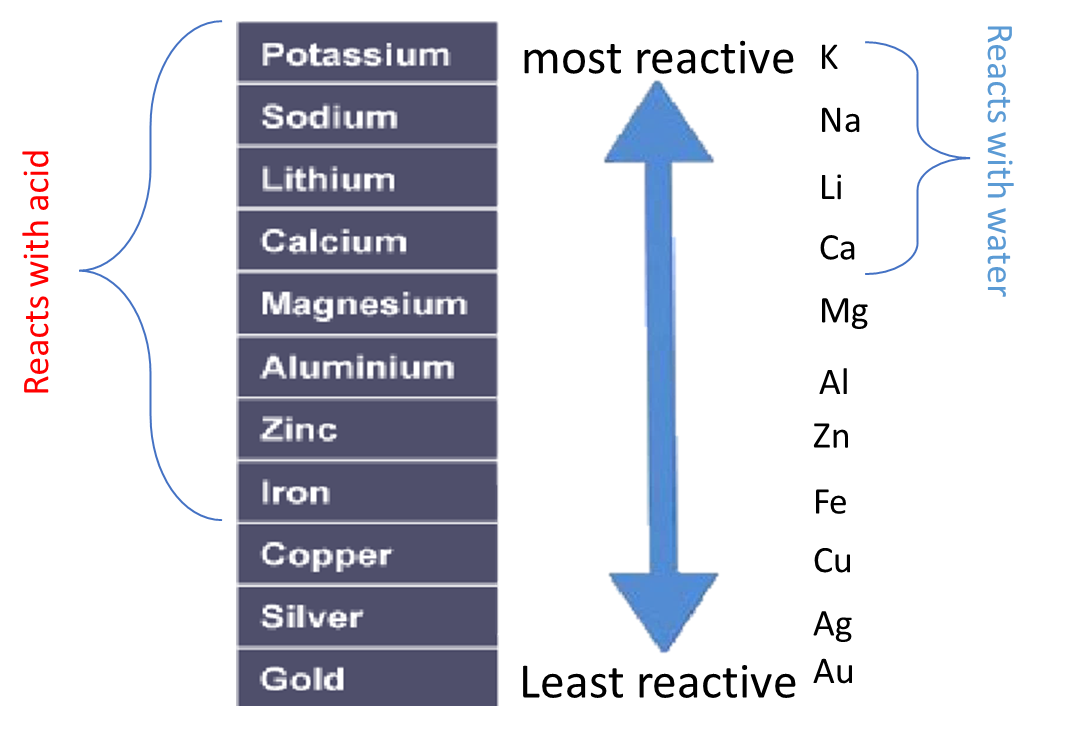
A metal will displace another metal from its oxide that is lower in the reactivity series. For example, a reaction with magnesium and copper (II) oxide will result in the magnesium displacing the copper from its oxide:
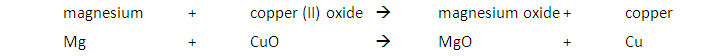
A metal will also displace another metal from its salt that is lower in the reactivity series. For example, the reaction between zinc and copper (II) sulfate solution will result in zinc displacing the copper from its salt:
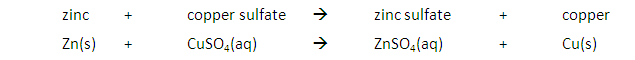
The blue colour of the copper (II) sulfate solution fades as colourless zinc sulfate solution is formed.
The thermite reaction is used to repair railways: the reaction between aluminium and iron oxide is highly exothermic and produces molten iron.
It should be properly controlled if demonstrated in the lab: watch this video and see how many mistakes in basic lab safety made by this teacher.
A more reactive metal will displace a less reactive metal.
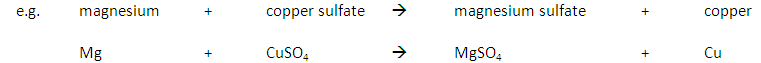
In addition a more reactive metal will react more vigorously than a less reactive metal.
For example, potassium takes a shorter time to react than sodium:
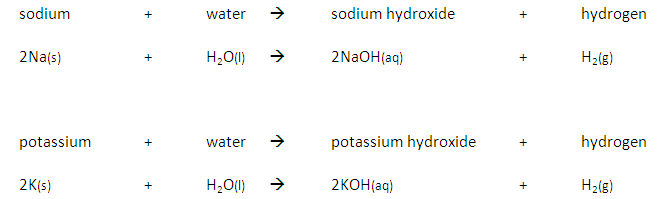
Iron rusts when oxygen and water are present.
Reaction:
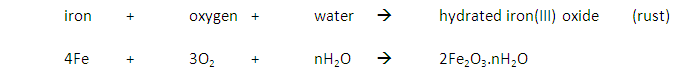
Barrier Methods: Rusting may be prevented by stopping the water and oxygen getting to the iron with a barrier of grease, oil, paint or plastic.
Galvanising: (coating in zinc) also prevents water and oxygen getting to the iron, but with galvanising even if the barrier is broken the more reactive zinc corrodes before the less reactive iron. During the process, the zinc loses electrons to form zinc ions.
Sacrificial Protection: Zinc blocks are attached to iron boat hulls and underground pipelines to act as sacrificial anodes. Zinc is more reactive than iron, so oxygen in the air reacts with the zinc to form a layer of zinc oxide instead of the iron.
This video shows the galvanising of pieces of steel to protect them from rusting:
Oxidation
Reduction
Redox: A reaction involving oxidation and reduction.
A good way to remember the definitions of oxidation and reduction in terms of electrons is:
Oxidising agent: A substance that gives oxygen or removes electrons (it is itself reduced).
Reducing agent: A substance that takes oxygen or gives electrons (it is itself oxidised).
Metals which are above hydrogen in the reactivity series will react with dilute hydrochloric or sulfuric acid to produce a salt and hydrogen.
metal + acid → salt + hydrogen
For example:
magnesium + hydrochloric acid → magnesium chloride + hydrogen
Mg (s) + 2HCl (aq) → MgCl₂ (aq) + H₂ (g)
This is a displacement reaction.
There is a rapid fizzing and a colourless gas is produced. This gas pops with a lighted splint, showing the gas is hydrogen.
The reaction mixture becomes warm as heat is produced (exothermic).
The magnesium disappears to leave a colourless solution of magnesium chloride.
If more reactive metals are used instead of magnesium the reaction will be faster so the fizzing will be more vigorous and more heat will be produced.
Most metals are found in the Earth’s crust combined with other elements. Such compounds are found in rocks called ore, rocks from which it is worthwhile to extract a metal.
A few very unreactive metals, such as gold, are found native which means they are found in the Earth’s crust as the uncombined element.
Extraction of a metal from its ore typically involves removing oxygen from metal oxides.
If the ore contains a metal which is below carbon in the reactivity series then the metal is extracted by reaction with carbon in a displacement reaction.
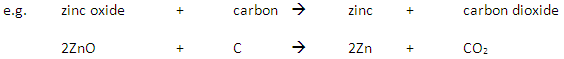
If the ore contains a metal which is above carbon in the reactivity series then electrolysis (or reaction with a more reactive metal) is used to extract the metal.
Extraction of a metal from its ore typically involves removing oxygen from metal oxides.
If the ore contains a metal which is below carbon in the reactivity series then the metal is extracted by reaction with carbon in a displacement reaction.
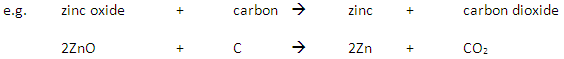
If the ore contains a metal which is above carbon in the reactivity series then electrolysis (or reaction with a more reactive metal) is used to extract the metal.
| Aluminium | |
|---|---|
| Use | Property |
| Aircrafts and cans | Low density / resists corrosion |
| Power cables | Conducts electricity / ductile |
| Pots and pans | Low density / strong (when alloyed) / good conductor of electricity and heat |
Aluminium resists corrosion because it has a very thin, but very strong, layer of aluminium oxide on the surface.
| Copper | |
|---|---|
| Use | Property |
| Electrical wires | very good conductor of electricity and ductile |
| Pots and pans | very good conductor of heat / very unreactive / malleable |
| Water pipes | unreactive / malleable |
| Surfaces in hospitals | antimicrobial properties / malleable |
| Iron | |
|---|---|
| Use | Property |
| Buildings | Strong |
| Saucepans | Conducts heat / high melting point / malleable |
| Steel | ||
|---|---|---|
| Type of steel | Iron mixed with | Some uses |
| Mild steel | up to 0.25% carbon | nails, car bodies, ship building, girders |
| High-carbon steel | 0.6%-1.2% carbon | cutting tools, masonry nails |
| Stainless steel | Chromium (and nickel) | cutlery, cooking utensils, kitchen sinks |
Mild steel is a strong material that can easily be hammered into various shapes (malleable). It rusts easily.
High-carbon steel is harder than mild steel but more brittle (not as malleable).
Stainless steel forms a strong, protective oxide layer so is very resistant to corrosion.
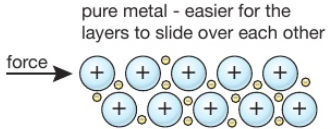
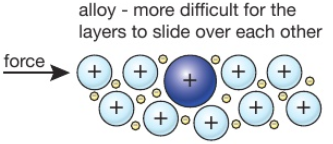
An alloy is a mixture of a metal with, usually, other metals or carbon.
For example, brass is a alloy of copper and zinc, and steel is an alloy of iron and carbon.
Alloys are harder than the individual pure metals from which they are made.
In an alloy, the different elements have slightly different sized atoms. This breaks up the regular lattice arrangement and makes it more difficult for layers of ions to slide over each other.
Indicators are substances which change colour in response to a change in pH (acid or alkali).
| Indicator | Colour in acidic solution [H+] | Colour in alkaline solution [OH-] |
|---|---|---|
| Litmus | Red | Blue |
| Methyl orange | Red | Yellow |
| Phenolphthalein | Colourless | Pink |
Methyl orange is orange in a neutral solution
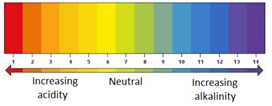
The pH scale ranges from 0 to 14, and tells you how acidic or how alkaline a solution is.
| strongly acidic | weakly acidic | neutral | weakly alkaline | strongly alkaline | |
|---|---|---|---|---|---|
| pH | 0-3 | 4-6 | 7 | 8-10 | 11-14 |
An indicator is a substance that has more than one colour form depending on the pH.
Universal indicator is a mixture of different dyes which change colour in a gradual way over a range of pH.
An acid is source of hydrogen ions (H+).
An alkali is source of hydroxide ions (OH–).
Metal oxides, metal hydroxides and ammonia (NH₃) are called bases.
Bases neutralise acids by combining with the hydrogen ions in them.
The key reaction is:
acid + base → salt + water
An example of this is:
sulfuric acid + copper oxide → copper sulfate + water
H₂SO₄ + CuO → CuSO₄ + H₂O
Titration is used to find out precisely how much acid neutralises a certain volume of alkali (or vice versa).
The diagram shows the titration method for a neutralisation reaction between hydrochloric acid and sodium hydroxide, using phenolphthalein as an indicator. The indicator changes colour when neutralisation occurs.
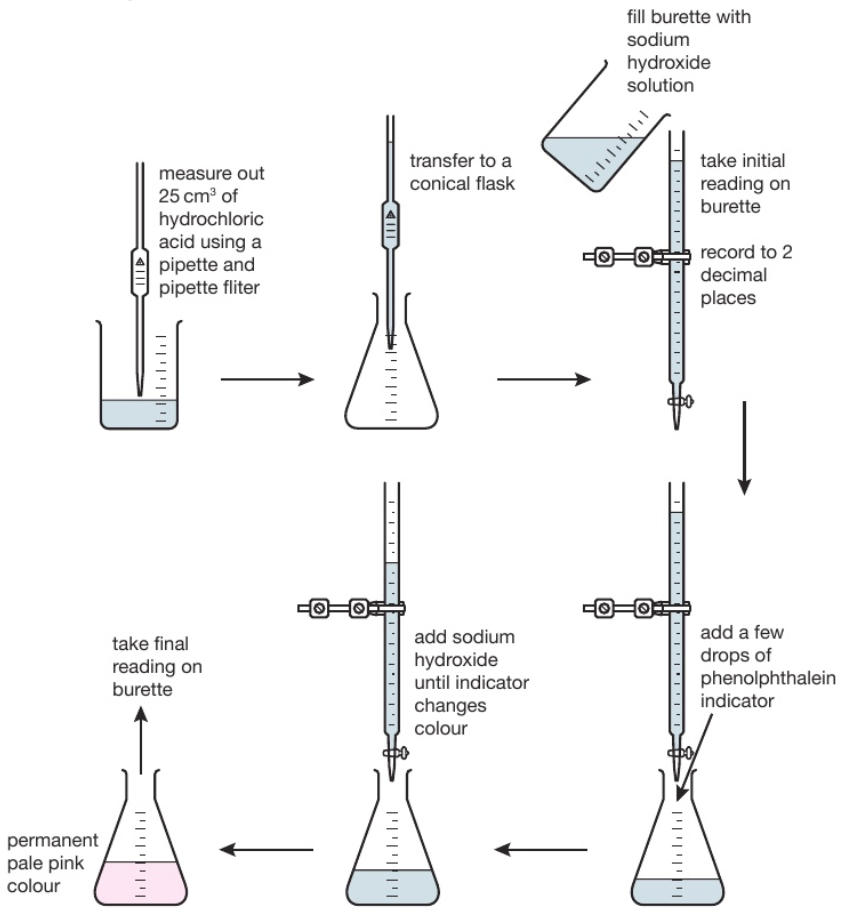
The conical flask is swirled to mix the solutions each time alkali is added. When reading the burette it is important to be aware that the numbers on the scale increase from top to bottom. Readings are usually recorded to the nearest 0.05cm³ so all readings should be written down with 2 decimal places. The second decimal place is given as a ‘0’ if the level of the solution is on a line, or ‘5’ if it is between the lines. The volume of alkali added is calculated by subtracting the final reading from the initial reading. Various indicators can be used such as phenolphthalein or methyl orange. However universal indicator should not be used since it has a wide range of colours rather than one specific colour change so it would be unclear when the precise endpoint of titration was achieved.
This process is repeated a number of times. The first time it is done roughly to get a good approximation of how much alkali needs to be added. On subsequent attempts, the alkali is added very slowly when approaching the correct volume.
Here are a couple of videos explaining how to perform titration calculations:
| Salt | Solubility | Exceptions |
|---|---|---|
| sodium (Na+), potassium (K+) and ammonium (NH4+) | soluble | none |
| nitrates (NO3-) | soluble | none |
| chlorides (Cl-) | soluble | silver chloride (AgCl) and lead (II) chloride (PbCl2) |
| sulfates (SO42-) | soluble | barium sulfate (BaSO4), calcium sulfate (CaSO4) and lead (II) sulfate (PbSO4) |
| carbonates (CO32-) | insoluble | sodium carbonate (Na2CO3), potassium carbonate (K2CO3) and ammonium carbonate ((NH4)2CO3) |
| hydroxides (OH-) | insoluble | sodium hydroxide (NaOH), potassium hydroxide (KOH) and calcium hydroxide (Ca(OH)2) (calcium hydroxide is slightly soluble) |
An acid is a proton (H⁺) donor.
A base is a proton (H⁺) acceptor.
A proton is the same as a hydrogen ion. A good way to think about that is to realise that a hydrogen atom is just one proton and zero neutrons surrounded by only one electron. If that atom becomes an ion by the removal of the electron, then only one proton is left.
When sulfuric acid reacts with copper (II) oxide (CuO):
Cu²⁺O²⁻ (s) + H₂SO₄ (aq) → Cu²⁺ (aq) + SO₄²⁻ (aq) + H₂O (l)
H₂SO₄ is an acid. It donates protons (H⁺) to CuO, the base.
An acid is a proton donor.
A base is a proton acceptor.
A proton is the same as a hydrogen ion. A good way to think about that is to realise that a hydrogen atom is just one proton and zero neutrons surrounded by only one electron. If that atom becomes an ion by the removal of the electron, then only one proton is left.
Acid reactions summary
alkali + acid → water + salt
base + acid → water + salt
carbonate + acid → water + salt + carbon dioxide
metal + acid → salt + hydrogen
To assist remembering this list, many pupils find it useful to remember this horrid looking but very effective mnemonic:
AAWS
BAWS
CAWS CoD
MASH
Acids are a source of hydrogen ions (H⁺) when in solution. When the hydrogen in an acid is replaced by a metal, the compound is called a salt. The name of the salt depends on the acid used. For example if sulfuric acid is used then a sulfate salt will be formed.
| Parent acid | Formula | Salt | Formula ion |
|---|---|---|---|
| sulfuric acid | H2SO4 | sulfate | SO42- |
| hydrochloric acid | HCl | chloride | Cl- |
| nitric acid | HNO3 | nitrate | NO3- |
Acid + Alkali and Acid + Base
A base is a substance that can neutralise an acid, forming a salt and water only.
Alkalis are soluble bases. When they react with acids, a salt and water is formed. The salt formed is often as a colourless solution. Alkalis are a source of hydroxide ions (OH⁻) when in solution.
alkali + acid → water + salt
base + acid → water + salt
Examples of acid + alkali reactions:
Example of an acid + base reaction:
CuO (s) + H₂SO₄ (aq) → CuSO₄ (aq) + H₂O (l)
Acid + Carbonate
carbonate + acid → water + salt + carbon dioxide
A carbonate is a compound made up of metal ions and carbonate ions. Examples of metal carbonates are sodium carbonate, copper carbonate and magnesium carbonate.
When carbonates react with acids, bubbling is observed which is the carbon dioxide being produced. If the acid is in excess the carbonate will disappear.
Examples of acid + carbonate reactions:
Acid + Metal
metal + acid → salt + hydrogen
Metals will react with an acid if the metal is above hydrogen in the reactivity series.
When metals react with acids, bubbling is observed which is the hydrogen being produced. If the acid is in excess the metal will disappear.
Examples of acid + metal reactions: