Preparation of CuSO₄ – video
Below is the preparation of copper sulfate crystals (CuSO4.5H2O) through the process of filtration and crystallisation
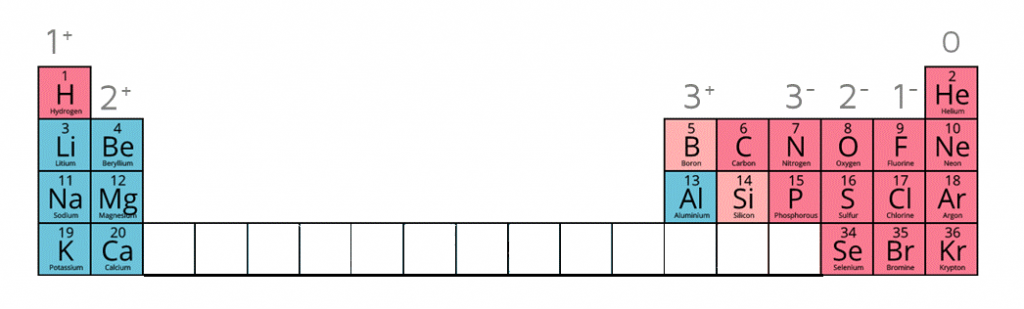
1:38b know the charges of these ions: metals in Groups 1, 2 and 3, non-metals in Groups 5, 6 and 7, hydrogen (H⁺), hydroxide (OH⁻), ammonium (NH₄⁺), carbonate (CO₃²⁻), nitrate (NO₃⁻), sulfate (SO₄²⁻)
| Name of Ion | Formula | Charge |
|---|---|---|
| Sulfate | SO42- | -2 |
| Carbonate | CO32- | -2 |
| Nitrate | NO3- | -1 |
| Hydroxide | OH- | -1 |
| Ammonium | NH4+ | +1 |
| Hydrogen ion | H+ | +1 |
Ion charges on the periodic table
Reactions with oxygen – video
Underneath are sodium, magnesium, iron, carbon, phosphorus, Sulphur burning in oxygen
2:19a understand how the rusting of iron may be prevented by: barrier methods, galvanising
Barrier Methods: Rusting may be prevented by stopping the water and oxygen getting to the iron with a barrier of grease, oil, paint or plastic.
Galvanising: (coating in zinc) also prevents water and oxygen getting to the iron, but with galvanising even if the barrier is broken the more reactive zinc corrodes before the less reactive iron. During the process, the zinc loses electrons to form zinc ions.
2:28a describe the use of litmus to distinguish between acidic and alkaline solutions
| Indicator | Colour in acidic solution | Colour in alkaline solution |
|---|---|---|
| Litmus | Red | Blue |
3 reactions of acid – video
Underneath are 3 acid reactions :
alkali + acid → water + salt
base + acid → water + salt
carbonate + acid → water + salt + carbon dioxide
Identifying cations using sodium hydroxide – video
Underneath is Sodium Hydroxide added to:
Iron (II) Ions
Iron (III) Ions
Copper (II) Ions
Ammonia
2:48a describe a test for CO₃²⁻ using hydrochloric acid and identifying the gas evolved
Describe tests for anions: Carbonate ions (CO32-)
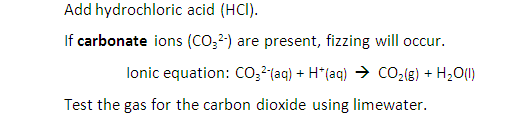
Test for Anions – video
Underneath are the tests for:
Carbonate ions
sulfate ions
chloride ions
bromide ions
iodide ions
4:02a understand how to represent organic molecules using molecular formulae, general formulae, structural formulae and displayed formulae
The molecular formula shows the actual number of atoms of each element in a molecule.
The general formula shows the relationship between the number of atoms of one element to another within a molecule. Members of a homologous series share the same general formula. The general formula for alkanes is CnH2n+2 and the general formula for alkenes is CnH2n.
A structural formula shows how the atoms in a molecule are joined together.
The displayed formula is a full structural formula which shows all the bonds in a molecule as individual lines.
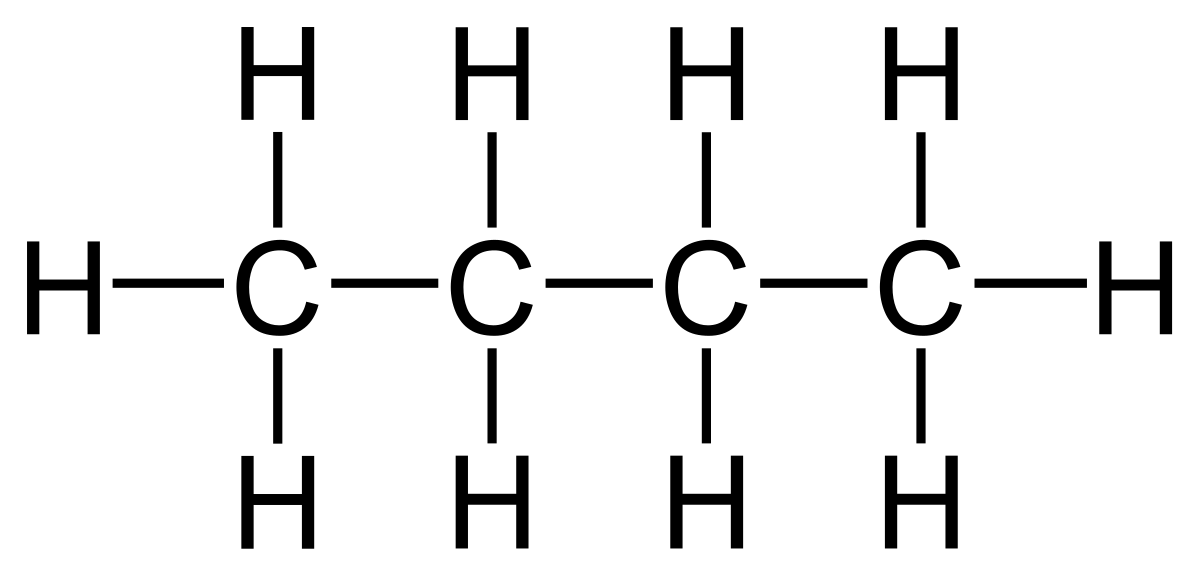
The terms above are demonstrated with the example of butane.
- Displayed formula:
- Molecular formula: C₄H₁₀
- General formula (alkanes): CnH2n+2
- Structural formula: CH₃ – CH₂ – CH₂ – CH₃
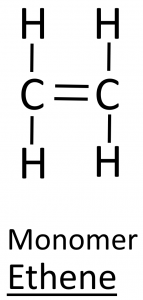
The terms above are demonstrated with the example of ethene, which contains a double bond.
- Displayed formula:
- Molecular formula: C₂H₄
- General formula (alkenes): CnH2n
- Structural formula: CH₂ = CH₂