1:14 know what is meant by the terms atom and molecule
Atom: An atom is the smallest part of an element.
Molecule: A molecule is made of a fixed number of atoms covalently bonded together.
Atom: An atom is the smallest part of an element.
Molecule: A molecule is made of a fixed number of atoms covalently bonded together.
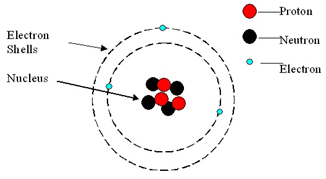
An atom consists of a central nucleus, composed of protons and neutrons.
This is surrounded by electrons, orbiting in shells (energy levels).
Atoms are neutral because the numbers of electrons and protons are equal.
| Mass | Charge | |
|---|---|---|
| Proton | 1 | +1 |
| Neutron | 1 | 0 |
| Electron | negligible (1/1836) | -1 |
Atomic number: The number of protons in an atom.
Mass number: The number of protons and neutrons in an atom.
Isotopes: Atoms of the same element (same number of protons) but with a different number of neutrons.
Relative atomic mass (Ar): The average mass of an atom compared to 1/12th the mass of carbon-12.
75% of chlorine atoms are the type 35Cl (have a mass number of 35)
25% of chlorine atoms are of the type 37Cl (have a mass number of 37)
In order to calculate the relative atomic mass (Ar) of chlorine, the following steps are used:
Example question:
A sample of bromine contained the two isotopes in the following proportions: bromine-79 = 50.7% and bromine-81 = 49.3%.
Calculate the relative atomic mass (Ar) of bromine.