2:48a describe a test for CO₃²⁻ using hydrochloric acid and identifying the gas evolved
Describe tests for anions: Carbonate ions (CO32-)
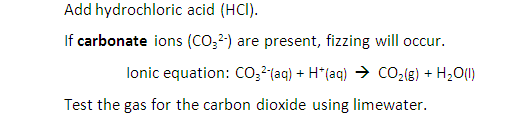
Describe tests for anions: Carbonate ions (CO32-)
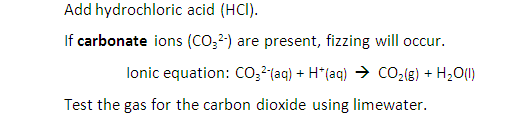
Describe tests for anions: Halide ions (Cl–, Br– and I–)
Underneath are the tests for:
chloride ion test, bromide ion test, iodide ion test, sulfate ion test, carbonate ion test

Describe tests for anions: Sulfate ions (SO42–)

Describe tests for anions: Carbonate ions (CO32-)
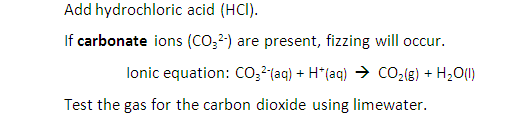
Add anhydrous copper (II) sulfate (CuSO4) to a sample.
If water is present the anhydrous copper (II) sulfate will change from white to blue.
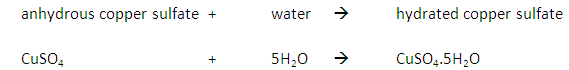
If the sample is pure water it will boil at 100oC