4:19 know the general formula for alkanes
Alkanes have the general formula CnH2n+2
This means that to work out the number of hydrogens, you double the number of carbons and then add two.
Alkanes have the general formula CnH2n+2
This means that to work out the number of hydrogens, you double the number of carbons and then add two.
Saturated: A molecule containing only single bonds between carbon atoms. For example, alkanes as described as saturated molecules.
Unsaturated: A molecule containing a carbon-carbon double or triple bond. For example, alkenes as described as unsaturated molecules.
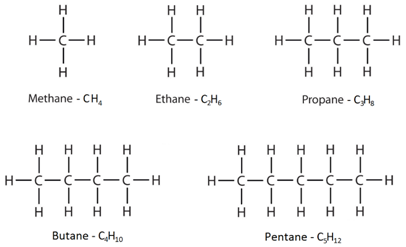
The displayed formulae show all the atoms and bonds drawn out.
The molecular formulae just show the number of each type of atom in the molecule.
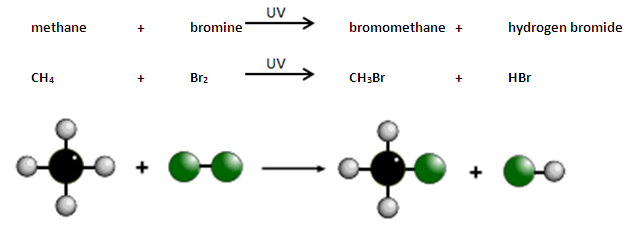
Alkanes react with bromine in the presence of UV light, e.g. sunlight.
A hydrogen atom in the alkane is replaced by a bromine atom.
This is known as substitution.