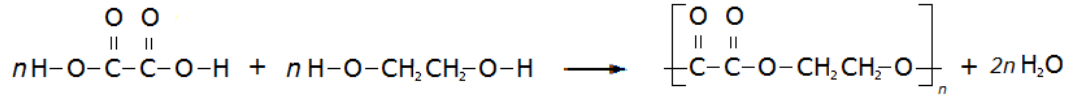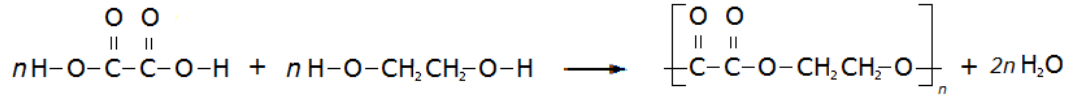Polyesters are polymers formed when two types of monomer join together alternately. Where each joins to the next a small molecule, such as water or hydrogen chloride, is lost. This is called a condensation polymerisation reaction.
One of the monomers is a diol, an alcohol with a -OH functional group at each end. An example is hexane-1,6-diol which has the structural formula CH₂OHCH₂CH₂CH₂CH₂CH₂OH and the displayed formula:
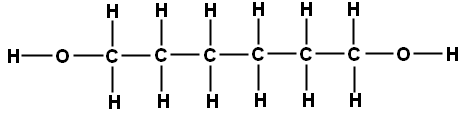
Since it is only the -OH functional groups which are important for polymerisation, this can we re-written with the central block of carbons represented as a block:

The other monomer is a dicarboxylic acid, a molecule with a -COOH functional group at each end. An example is hexane-1,6-dioic acid which has the structural formula HOOCCH₂CH₂CH₂CH₂COOH and the displayed formula:
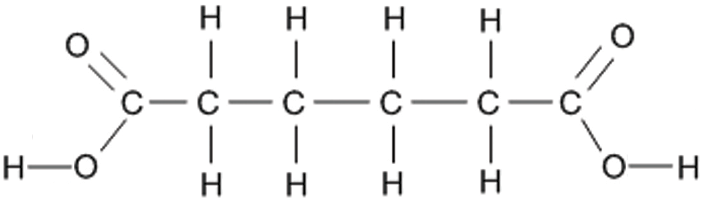
Since it is only the -COOH functional groups which are important for polymerisation, this can we re-written with the central block of 4 carbons represented as a block:
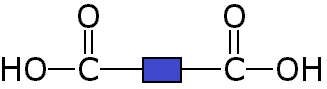
These two different types of monomer (the diol and the dicarboxylic acid) can join to form a polymer with the loss of a water molecule at every bond. As above, this can be simplified by only looking at the functional groups and representing the other carbons as blocks, so the whole process looks like:
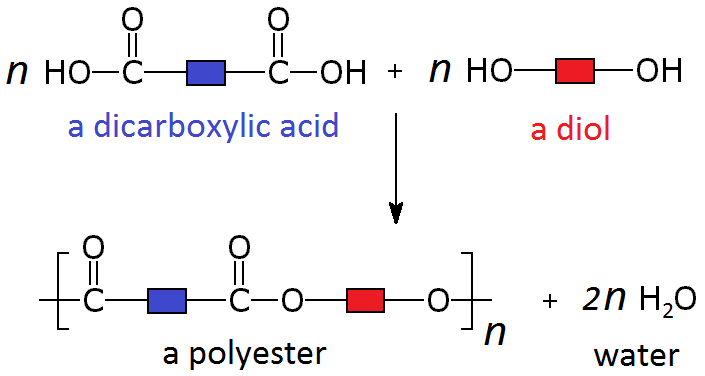
A simple example of this is the condensation polymerisation reaction between ethanedioic acid and ethandiol: