3.20 describe the role of total internal reflection in transmitting information along optical fibres and in prisms
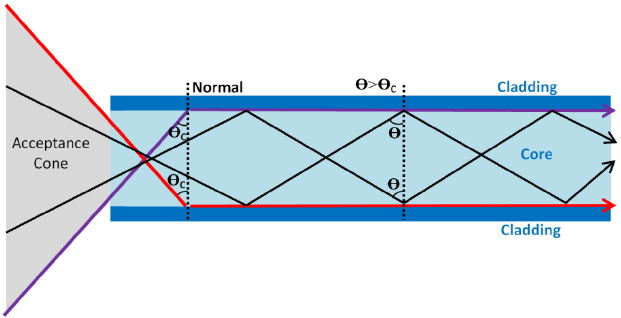
Total Internal Reflection:
- Used to transmit signals along optical fibres.

Total Internal Reflection:
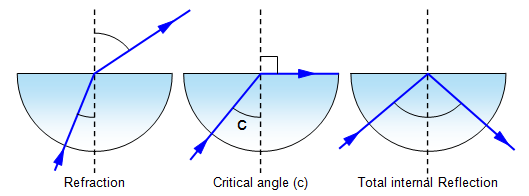
Critical Angle:
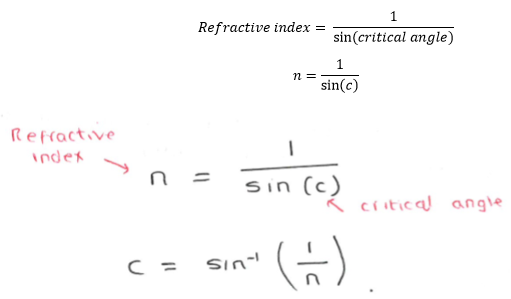
also remember:
critical angle = sin-1(1/n)
sound waves are longitudinal waves which can be reflected and refracted.
the frequency range for human hearing is 20–20 000 Hz
|
With the microphone plugged into the oscilloscope and a sound incident on the microphone, the microphone will transfer the sound into an electrical signal which the oscilloscope can display .The x axis show the time base which can be adjusted for example 2ms for 1 square so time period and frequency can be calculated from this, along the y axis voltage is displayed as the wave is converted into an electrical signal this means amplitudes can be compared. |
|
High frequency means high pitch. If a string vibrates with a higher frequency then the note sounds higher. |
The greater the amplitude the louder the sound. Bigger vibrations of a sting mean more energy is being put in so more energy out as sound waves.
know the units for
Mass = kilogram (kg)
energy = joule (J)
velocity = metre/second (m/s)
acelleration = metre/ second 2 (m/s2)
force = newton (N)
time = second (s)
power = watt (W)
|
Energy Stores: Chemical – e.g. the food we eat Kinetic – movement energy Gravitational – objects that are lifted up Elastic – e.g. from springs Thermal – from hot objects Magnetic – objects in magnetic fields Electrostatic – charged objects Nuclear – stored within a nucleus
|
In any process energy is never created or destroyed. (It is just transferred from one store to another.)
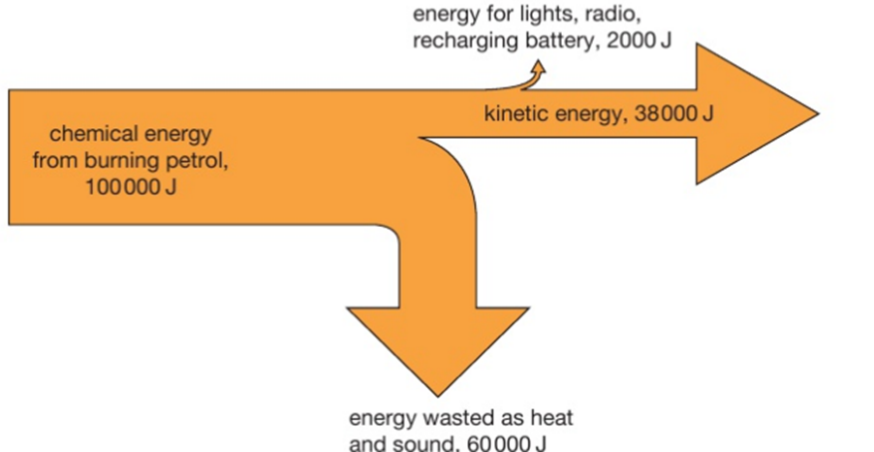
The energy flow is shown by arrows whose width is proportional to the amount of energy involved. The wasted and useful energy outputs are shown by different arrows.
Conduction is the transfer of thermal energy through a substance by the vibration of the atoms within the substance. Metals are good conductors because they have free electrons that can move easily through the metal, making the transfer of energy happen faster.
Convection occurs in a liquid or gas. These expand when heated because the particles move faster and take up more volume – the particles remain the same size but become further apart. The hot liquid or gas is less dense, so it rises into colder areas. The denser, colder liquid or gas falls into the warm areas. In this way, convection currents are set up which transfer heat from place to place.
Thermal radiation is the transfer of energy by infrared (IR) waves. These travel very quickly in straight lines.
|
– Light, shiny surfaces are good reflectors of IR and so are poor at absorbing it. – Dark, matt surfaces are poor reflectors and good at absorbing IR. – This means that placed next to a heat source, a dark object would heat up faster than a light one. – Dark matt surfaces are also best at emitting IR. This means that a hot object with a light shiny surface will emit less IR than a dark matt object at the same temperature. – Hotter objects emit more IR per second. The type of EM wave emitted also changes with temperature – the higher the temperature the higher the frequency of EM wave emitted. |
A good insulating material is a poor conductor that contains trapped air, e.g. foam, feathers, glass fibre. Being a poor conductor (non-metal) prevents heat transfer by conduction and the trapped air prevents convection currents.
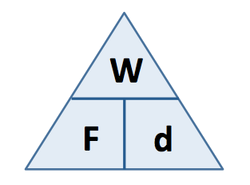
Work Done (J) = Force (N) x distance moved (m)
|
Work done = energy transferred |
Gravitational potential energy (J) = Mass (kg) x gravitational field strength (N/kg) x height (m)
Kinetic energy (J) = 0.5 x mass (kg) x velocity (m/s) 2
Because energy is conserved the decrease in GPE = increase in KE, for a falling object if no energy is lost to the surroundings
power is the rate of transfer of energy, or the rate of work done. so p = E/t
The units for:
temperature: degree Celsius (°C) or Kelvin (K)
Energy: Joule (J)
mass: Kilogram (kg)
density: kilogram/metre cubed (kg/m3)
distance: metre (m)
area: metre squared (m2)
volume: metre cubed (m3)
velocity: metre per second (m/s)
acceleration: metre per second squared (m/s2)
force: newton (N)
pressure: pascal (Pa)
the unit for
specific heat capacity: joules/kilogram degree Celsius (J/kg °C)
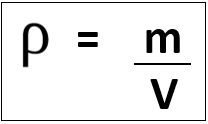
Units of density depend on units used for mass and volume:
E.g. mass in [g] and volume in [cm3] gives density in [g/cm3], however mass in [kg] and volume in [m3] gives density in [kg/m3].
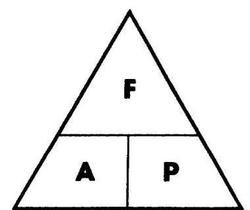
pressure (Pa) = force (N)/ area (m2)
Pressure in liquids:
Pressure in liquids acts equally in all directions as long as the liquid is not moving.
Pressure difference [Pa] = Density [kg/m3] x g [N/kg] x Height [m]
ΔP = ρ g h
P1 – Patm = ρ g h
P1 = ρ g h + Patm
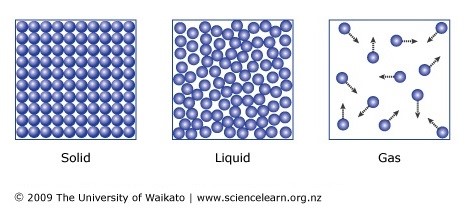
solids:
liquids:
gasses:
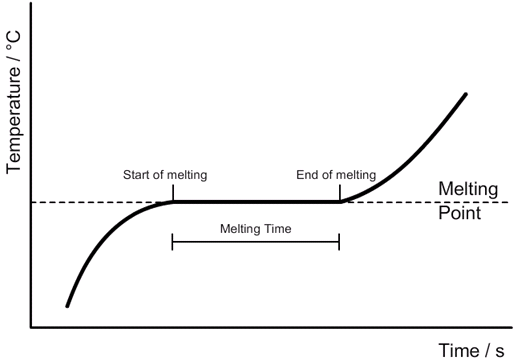
Specific heat capacity:
Change in thermal energy [J] = Mass [kg] x Specific heat capacity [J/kg 0C] x Change in temperature [0C]
Gas laws:
Absolute zero:
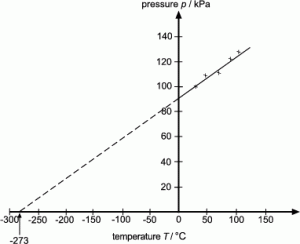
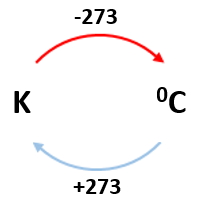
0 K = -273 0C
E.g. 100K = -1730C
2000C = 473K
As you increase the temperature of a gas, the kinetic energy of the gas particles increases and thus their average speed also increases.
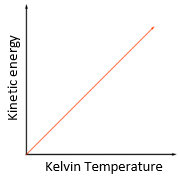
The Kelvin temperature of a gas is proportional to the average kinetic energy of its molecules.
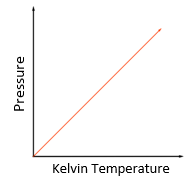
P1/T1 = P2/T2
*Temperature must be in Kelvin
Temperature law:
For a fixed mass of gas at constant volume, the pressure is directly proportional to the Kelvin temperature