Writing ionic formulae – Tyler de Witt video
This excellent video from the lovely Tyler de Witt explains how to write the formula of an ionic compound:
This excellent video from the lovely Tyler de Witt explains how to write the formula of an ionic compound:
This video shows how to work out the formula of a compound which contains polyatomic ions:
These videos are a really good introduction to ionic bonding:
These videos are a good introduction to covalent bonding:
This excellent Tyler de Witt video addresses a common mistake around diatomic elements (H2, N2, O2, F2, Cl2, Br2, I2).
For example, if chlorine is in its elemental form (i.e. not bonded in a compound) the formula is Cl2, but when chlorine is bonded to sodium the formula of sodium chloride is NaCl.
This video explains the structure of alloys, including different forms of steel.
Note that a slight improvement could be made in the explanation: the structure of metals should be described as “layers of metal IONS in a sea of delocalised electrons”.
Although quite long, this Tyler de Witt video is a good summary of Electrolysis.
The awesome Professor Sir Martyn Poliakoff shows us the reaction of some alkali metals and water.
Here is a great video on Fluorine, which is the most reactive halogen (group 7):
The reactions of various halogens with iron wool is a great way to see how reactive one halogen is compared to another:
Special thanks to Charlie R for suggesting I add a video on halogen displacement.
Underneath are sodium, magnesium, iron, carbon, phosphorus, Sulphur burning in oxygen
This video shows the reaction with oxygen of various elements:
The thermite reaction is used to repair railways: the reaction between aluminium and iron oxide is highly exothermic and produces molten iron.
It should be properly controlled if demonstrated in the lab: watch this video and see how many mistakes in basic lab safety made by this teacher.
This video shows the galvanising of pieces of steel to protect them from rusting:
Here are a couple of videos explaining how to perform titration calculations:
Underneath are 3 acid reactions :
alkali + acid → water + salt
base + acid → water + salt
carbonate + acid → water + salt + carbon dioxide
Underneath is Sodium Hydroxide added to:
Iron (II) Ions
Iron (III) Ions
Copper (II) Ions
Ammonia
Underneath are the tests for:
Carbonate ions
sulfate ions
chloride ions
bromide ions
iodide ions
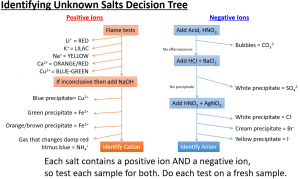
Here is summary of “testing for ions” (otherwise known as qualitative analysis). These “tests for ions” are commonly asked in iGCSE Edexcel Chemistry.
Each ionic compound contains positive ions and negative ions. To use the decision tree, start at the top and if a test is negative then move down to the next test.
Here are a couple of videos explaining how to do bond energy calculations:
Here is a video introducing reversible reactions:
This video explains how the position of equilibrium in a reversible reaction is affected by changes to temperature.
This video explains how the position of equilibrium in a reversible reaction is affected by changes to pressure.
This video shows the addition reaction between bromine and an alkene.
The observation from the reaction is the colour change from orange to colourless.
This video does not quite use the right language for the various fractions as appropriate to the Edexcel iGCSE, but it is nevertheless a good description of the process. Make sure you use the notes on tutorMyself.com to get the exact language you will need for your exam.
And another somewhat older video showing the industrial process of fractional distillation: