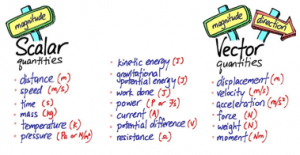
Solid
Arrangement: Particles are close together and regularly packed.
Movement: Particles vibrate around a fixed point.
Energy: Particles have less kinetic energy than both liquids and gasses.
Liquid

Arrangement: Particles are close together but irregular.
Movement: Particles are free to move.
Energy: Particles have less kinetic energy than gasses but more than solids.
Gas
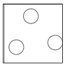
Arrangement: Particles are far apart and there are no forces between them.
Movement: Particles are free to move.
Energy: Particles have more kinetic energy than liquids and solids.