1:36 practical: know how to determine the formula of a metal oxide by combustion (e.g. magnesium oxide) or by reduction (e.g. copper(II) oxide)
To determine the formula of a metal oxide by combustion.
Example: When magnesium is burned in air, it reacts with oxygen (O2) to form magnesium oxide (MgO).
2Mg + O2 –> 2MgO
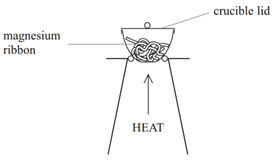
Method:
- Weigh a crucible and lid
- Place the magnesium ribbon in the crucible, replace the lid, and reweigh
- Calculate the mass of magnesium
- Heat the crucible with lid on until the magnesium burns (lid prevents magnesium oxide escaping therefore ensuring accurate results)
- Lift the lid from time to time (this allows air to enter)
- Stop heating when there is no sign of further reaction
- Allow to cool and reweigh
- Repeat the heating, cooling and reweigh until two consecutive masses are the same (this ensures all Mg has reacted and therefore the results will be accurate)
- Calculate the mass of magnesium oxide formed (mass after heating – mass of crucible)
It is then possible to use this data to calculate the empirical formula of the metal oxide.
To determine the formula of a metal oxide by reduction
Example: When copper (II) oxide is heated in a stream of methane, the oxygen is removed from the copper (II) oxide, producing copper, carbon dioxide and water:
4CuO + CH4 –> 4Cu + CO2 + 2H2O
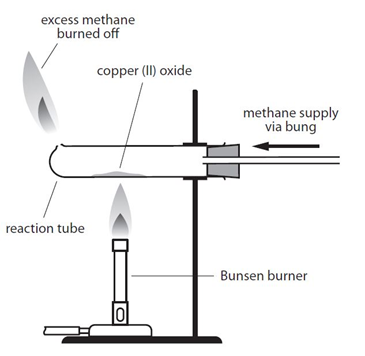
By comparing the mass of copper produced with the mass of copper oxide used it is possible to determine the formula of the copper oxide.
As an alternative, hydrogen gas could be used instead of methane:
CuO + H2 –> Cu + H2O
It is then possible to use this data to calculate the empirical formula of the metal oxide.
There is an important safety point to note with both versions of this experiment. Both methane and hydrogen are explosive if ignited with oxygen. It is important that a good supply of the the gas is allowed to fill the tube before the gas it lit. This flushes out any oxygen from the tube, so the gas will only burn when it exits the tube and comes into contact with oxygen in the air.