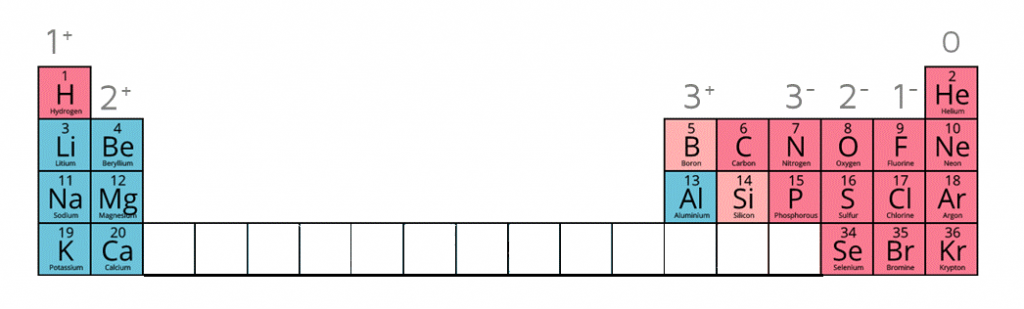
1:38a understand how to use the charges of these ions in ionic formulae: metals in Groups 1, 2 and 3, non-metals in Groups 5, 6 and 7, Ag⁺, Cu²⁺, Fe²⁺, Fe³⁺, Pb²⁺, Zn²⁺, hydrogen (H⁺), hydroxide (OH⁻), ammonium (NH₄⁺), carbonate (CO₃²⁻), nitrate (NO₃⁻), sulfate (SO₄²⁻)
When given this information of the following ions, it is possible to work out the formulae of ionic compounds which include these ions.
| Name of Ion | Formula | Charge |
|---|---|---|
| Sulfate | SO42- | -2 |
| Carbonate | CO32- | -2 |
| Nitrate | NO3- | -1 |
| Hydroxide | OH- | -1 |
| Ammonium | NH4+ | +1 |
| Silver ion | Ag+ | +1 |
| Zinc ion | Zn2+ | +2 |
| Hydrogen ion | H+ | +1 |
| Copper (II) ion | Cu2+ | +2 |
| Iron (II) ion | Fe2+ | +2 |
| Iron (III) ion | Fe3+ | +3 |
| Lead (II) ion | Pb2+ | +2 |
Ion charges on the periodic table