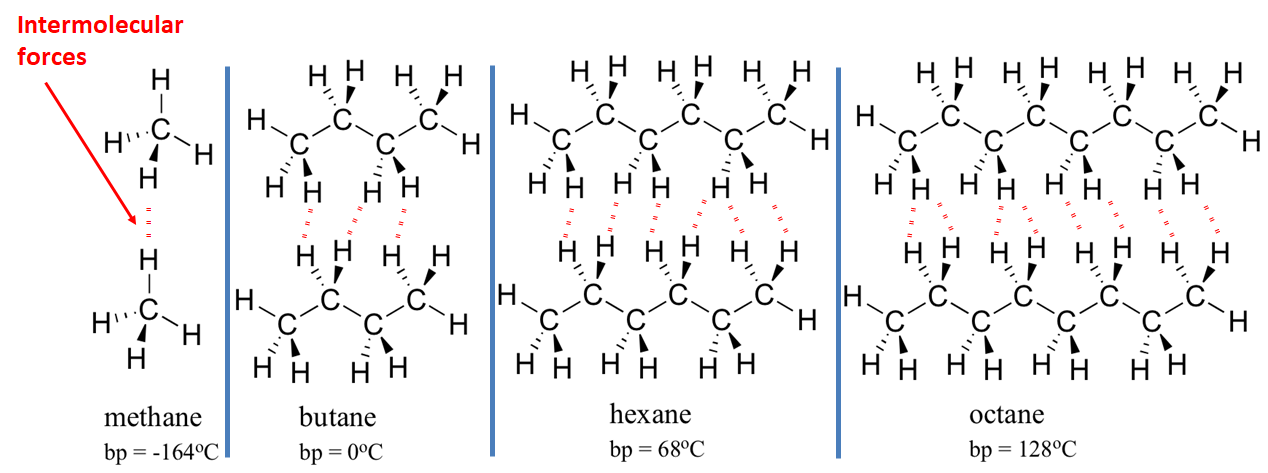
1:48 explain why the melting and boiling points of substances with simple molecular structures increase, in general, with increasing relative molecular mass
Larger molecules tend to have higher boiling points.
This is because larger molecules (molecules with more mass) have more forces of attraction between them. These forces, although weak, must be overcome if the substance is to boil, and larger molecules have more attractions which must be overcome.