1:58 (Triple only) describe experiments to investigate electrolysis, using inert electrodes, of molten compounds (including lead(II) bromide) and aqueous solutions (including sodium chloride, dilute sulfuric acid and copper(II) sulfate) and to predict the products
Electrolysis: The breaking down of a substance caused by passing an electric current through an ionic compound which is molten or in solution. New substances are formed.
ELECTROLYSIS OF MOLTEN IONIC COMPOUNDS
Example: The electrolysis of molten lead bromide (PbBr2)
-
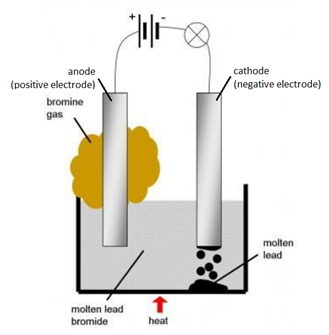
Solid lead bromide is heated and becomes molten. Explanation: ions become free to move.
- Electrodes attached to a power source are placed in the molten lead bromide. Explanation: these electrodes are made of either graphite or platinum because both conduct electricity and are fairly unreactive.
- From the diagram, the left-hand electrode becomes positively charged, this is called the anode. The right-hand becomes negatively charged, this is called the cathode. Explanation: delocalised electrons flow from the anode to the cathode.
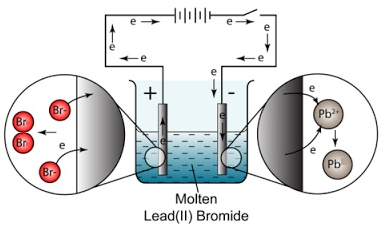
- At the anode a brown gas is given off. This is bromine gas (Br2(g)). Explanation: Negatively charged bromide ions are attracted to the anode (positive electrode). At the anode, bromide ions lose electrons (oxidation) and become bromine molecules.
- At the cathode a shiny substance is formed. This is molten lead (Pb(l) ). Explanation: Positively charged lead ions are attracted to the cathode (negative electrode). At the cathode, lead ions gain electrons (reduction) and become lead atoms.
Overall Reaction
word equation: lead bromide –> lead + bromine
chemical equation: PbBr2(l) –> Pb(l) + Br2(g)
Remember
OILRIG : Oxidation Is the Loss of electrons and Reduction Is the Gain of electrons
PANCAKE : Positive Anode, Negative Cathode
ELECTROLYSIS OF IONIC SOLUTIONS
Rules for working out elements formed from electrolysis of solutions
Follow these rules to decide which ions in solution will react at the electrodes:
At the cathode
Metal ions and hydrogen ions are positively charged. Whether you get the metal or hydrogen during electrolysis depends on the position of the metal in the reactivity series:
- The metal will be produced if it is less reactive than hydrogen
- Hydrogen gas (H2) will be produced if the metal is more reactive than hydrogen
At the anode
At the anode, the product of electrolysis is always oxygen gas (O2) unless the solution contains a high concentration of Cl–, Br- or I– ions, in which case a halogen is produced, e.g. chlorine gas (Cl2), bromine gas (Br2), and iodine gas (I2).
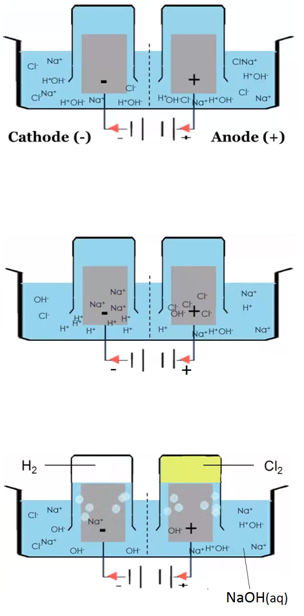
The electrolysis of sodium chloride solution (NaCl(aq))
-
Solid sodium chloride is dissolved in water. Explanation: The sodium ions and chloride ions become free to move.
- The solution also contains hydrogen ions (H+) and hydroxide ions (OH–). Explanation: Water is a very weak electrolyte. It ionises very slightly to give hydrogen ions and hydroxide ions:
H2O(l) ⇋ H+(aq) + OH–(aq)
- Chloride ions (Cl–) and hydroxide ions (OH–) are attracted to the anode.
- Sodium ions (Na+) and hydrogen ions (H+) are attracted to the cathode.
- At the anode a green gas is given off. This is chlorine gas (Cl2(g)). Explanation: chloride ions lose electrons (oxidation) and form molecules of chlorine. The chloride ions react at the anode instead of the hydroxide ions because the chloride ions are in higher concentration. The amount of chlorine gas produced might be lower than expected because chlorine is slightly soluble in water.
Electron half equation: 2Cl–(aq) –> Cl2 (g) + 2e–
- At the cathode a colourless gas is given off. This is hydrogen gas (H2(g)). Explanation: hydrogen ions gain electrons (reduction) and form molecules of hydrogen. The hydrogen ions react at the cathode because hydrogen is below sodium in the reactivity series.
Electron half equation: 2H+(aq) + 2e– –> H2 (g)
- The solution at the end is sodium hydroxide (NaOH(aq)).
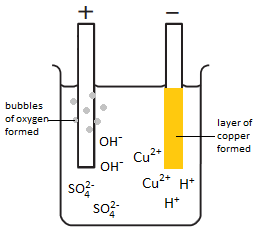
The electrolysis of copper sulfate solution (CuSO4(aq))
- Copper sulfate solution is composed of copper ions (Cu2+), sulfate ions (SO42-), hydrogen ions (H+) and hydroxide ions (OH–).
- At the cathode a brown layer is formed. This is copper. Explanation: copper ions gain electrons (reduction) and form atoms of copper. The copper ions react at the cathode instead of hydrogen ions because copper is below hydrogen in the reactivity series.
Electron half-equation: Cu2+(aq) + 2e– –> Cu (s)
- At the anode, bubbles of gas are given off. This is oxygen gas (O2(g)). Explanation: hydroxide ions lose electrons (oxidation) and form molecules of oxygen and water. The hydroxide ions react at the anode instead of the sulfate ions because the hydroxide ions are less stable.
Electron half-equation: 4OH–(aq) –> O2 (g) + 2H2O(l) + 4e–
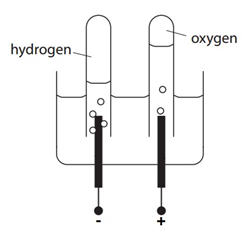
The electrolysis of sulfuric acid (H2SO4(aq))
- Sulfuric acid is composed of sulfate ions (SO42-), hydrogen ions (H+) and hydroxide ions (OH–).
- At the cathode bubbles of gas are formed. This is hydrogen gas (H2(g)). Explanation: hydrogen ions gain electrons (reduction) and form molecules of hydrogen.
Electron half-equation: 2H+(aq) + 2e– –> H2(g)
- At the anode, bubbles of gas are given off. This is oxygen gas (O2(g)). Explanation: hydroxide ions lose electrons (oxidation) and form molecules of oxygen and water. The hydroxide ions react at the anode instead of the sulfate ions because the hydroxide ions are less stable.
Electron half-equation: 4OH–(aq) –> O2 (g) + 2H2O(l) + 4e–
- Twice the volume of hydrogen gas is produce compared to oxygen gas. Explanation: from the two half equations, O2 needs 4e– but H2 only needs 2e– as can be seen from the equation
2H2O(l) –> 2H2(g) + O2(g)
There are twice the amount (in moles) of H2 compared to O2