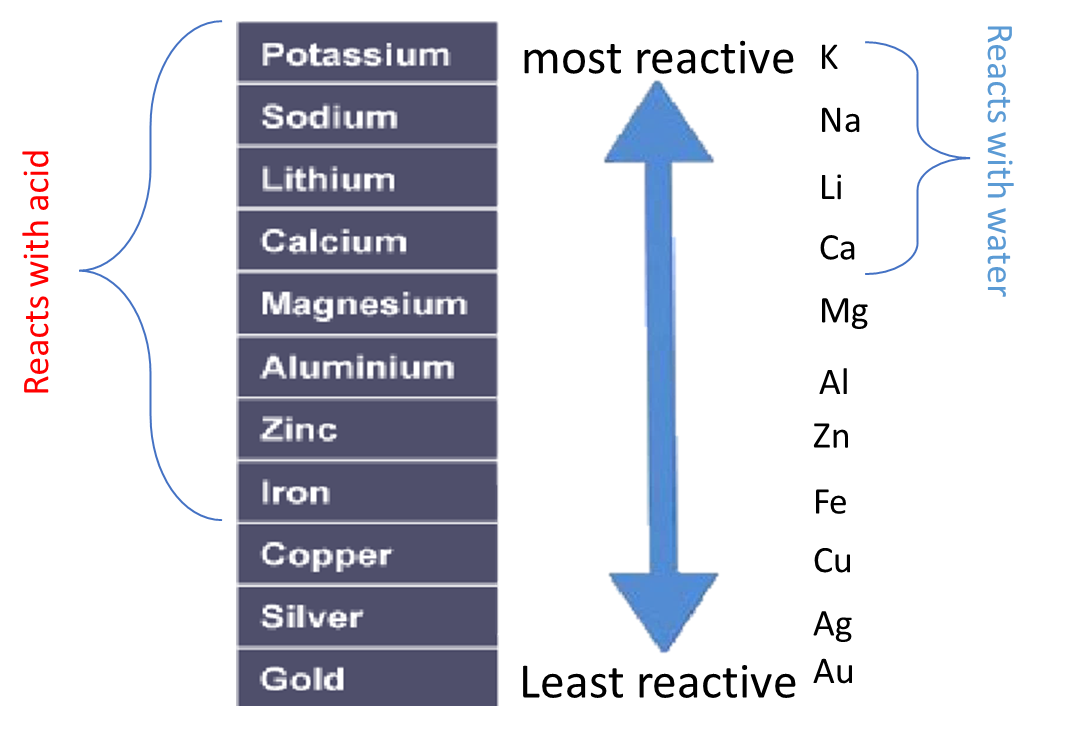
2:15 understand how metals can be arranged in a reactivity series based on their reactions with: water and dilute hydrochloric or sulfuric acid
 Some metals are more reactive than others.
Some metals are more reactive than others.
The order of reactivity can be determined by adding acid to different metals and observing the rate of reaction.
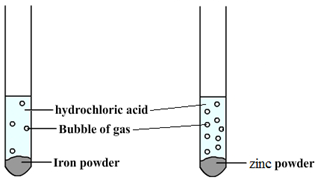
For example, when hydrochloric acid is added to iron (Fe) then bubbles of hydrogen are produced slowly. However, if the same acid is added to zinc (Zn) then bubbles will be produced more quickly. This tells us that zinc is more reactive than iron.
Instead of using acid, water can be used to test the relative reactivity of metals. However, many metals are too low in the reactivity series to react with water