3:14 (Triple only) draw and explain reaction profile diagrams showing ΔH and activation energy
Below is a diagram showing the reaction profile for the reaction of hydrogen with oxygen, which is EXOTHERMIC:
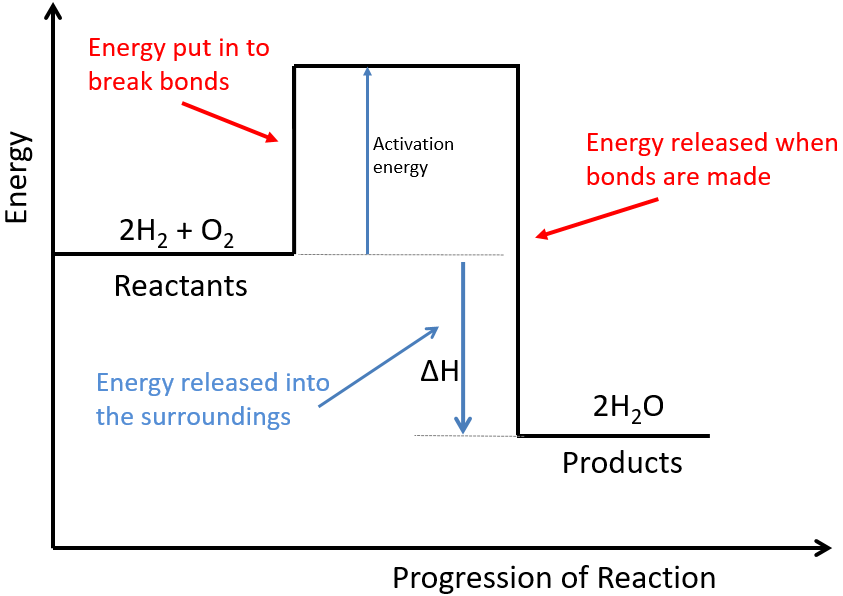
The activation energy is the minimum amount of energy required to start the reaction.
For an exothermic reaction, the products have less energy than the reactants. The difference between these energy levels is ΔH.
For an exothermic reaction, more energy is released when bonds are formed than taken in when bonds are broken.
Below is a diagram showing the reaction profile for the thermal decomposition of calcium carbonate, which is ENDOTHERMIC:
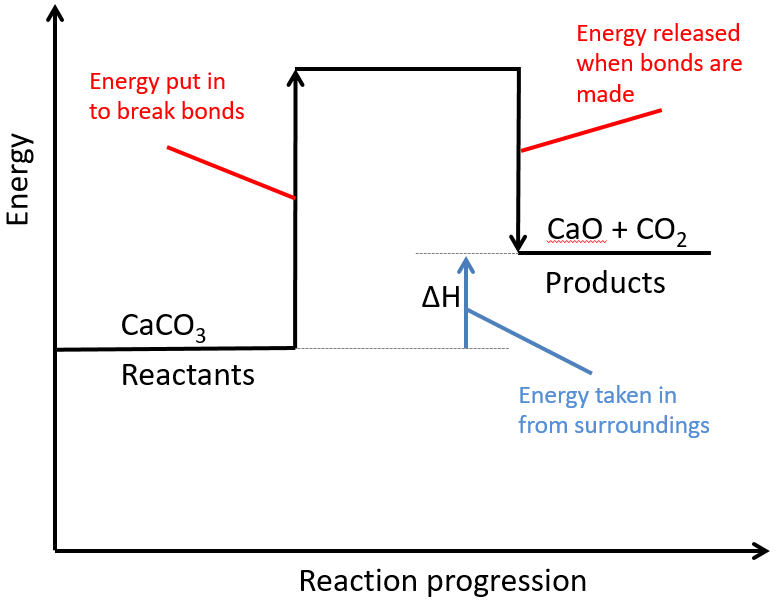
The activation energy is the minimum amount of energy required to start the reaction.
For an endothermic reaction, the products have more energy than the reactants. The difference between these energy levels is ΔH.
For an endothermic reaction, more energy is taken in to break bonds than is released when new bonds are formed.