3:16 practical: investigate the effect of different solids on the catalytic decomposition of hydrogen peroxide solution
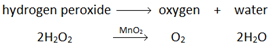
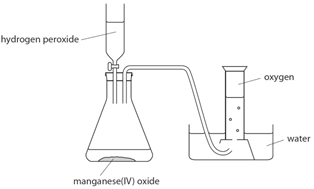 Oxygen (O2) is made in the lab from hydrogen peroxide (H2O2) using manganese(IV) oxide (MnO2) as a catalyst.
Oxygen (O2) is made in the lab from hydrogen peroxide (H2O2) using manganese(IV) oxide (MnO2) as a catalyst.
Different catalysts could be used to investigate which is the most effective in decomposing hydrogen peroxide. Examples of other substances which could be tested are:
- Manganese dioxide
- Liver
- Potato
- Potassium iodide
- Copper oxide
- Sodium chloride
Only some of these are effective catalysts when used with hydrogen peroxide. If a substance is not a catalyst, there will be no bubbles of oxygen produced. For other substances, such as liver which is a very effective catalyst in the decomposition of hydrogen peroxide, bubbles of oxygen will be produced quickly.