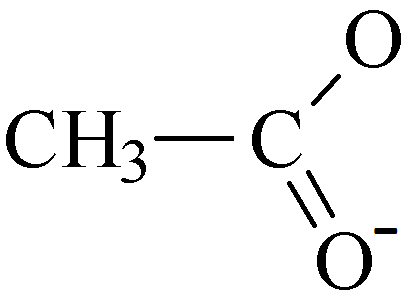4:36 (Triple only) describe the reactions of aqueous solutions of carboxylic acids with metals and metal carbonates
Dilute carboxylic acids react with metals in the same way as other dilute acids (e.g. hydrochloric acid) only more slowly.
For example, dilute ethanoic acid reacts with magnesium with a lot of fizzing to produce a salt and hydrogen, leaving a colourless solution of magnesium ethanoate:
magnesium + ethanoic acid → magnesium ethanoate + hydrogen
Mg (s) + 2CH₃COOH (aq) → (CH₃COO)₂Mg (aq) + H₂ (g)
Dilute carboxylic acids react with metal carbonates as they do with other acids, to give a salt, carbon dioxide and water.
For example, dilute ethanoic acid reacts with sodium carbonate with a lot of fizzing to produce a salt, carbon dioxide and water, leaving a colourless solution of sodium ethanoate:
sodium carbonate + ethanoic acid → sodium ethanoate + carbon dioxide + water
Na₂CO₃ (s) + 2CH₃COOH (aq) → 2CH₃COONa (aq) + CO₂ (g) + H₂O (l)
As can be seen in the examples above the charge on the ethanoate ion is -1.