Spec point which is NOT in Triple Science
3.05 know and use the relationship between the speed, frequency and wavelength of a wave: v = f × λ
wave speed (m/s) = frequency (Hz) x Wavelength (m)
Spec point which is NOT in Triple Science
wave speed (m/s) = frequency (Hz) x Wavelength (m)
Frequency (Hz) = 1/ Time Period (s)
You will need to use any of the in 3.05 and 3.06 to solve problems to do with sound waves and electromagnetic (light) waves
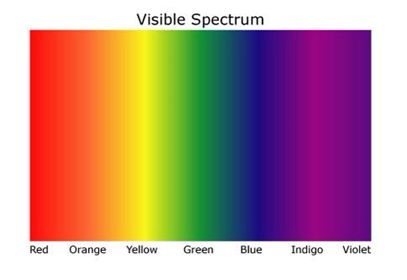
Electromagnetic Spectrum:
Radio Waves
Microwaves
Infrared (IR)
Visible Light
Ultraviolet (UV)
X – Rays
Gamma Rays
these are written in order of increasing frequency, lowest at the top
and decreasing wavelength, lowest at the bottom.
the colours displayed are in order of lowest frequency to the left highest frequency to the right.
|
uses of electromagnetic radiations, including: |
the detrimental effects of excessive exposure of the human body to electromagnetic waves:
• microwaves: internal heating of body tissue
• infrared: skin burns
• ultraviolet: damage to surface cells and blindness
• gamma rays: cancer, mutation
to reduce the risks:
light is a transverse wave that can be reflected and refracted
|
1. Set up your apparatus as shown in the diagram using a rectangular block. 2. Shine the light ray through the glass block 3. Use crosses to mark the path of the ray. 4. Join up crosses with a ruler 5. Draw on a normal where the ray enters the glass block 6. Measure the angle of incidence and the angle of refraction and add these to your results table 7. Comment on how the speed of the light has changed as the light moves between the mediums. 8. Repeat this for different angles of incidence and different glass prisms. |
|
1. Set up your apparatus as shown in the diagram using a rectangular block. 2. Shine the light ray through the glass block 3. Use crosses to mark the path of the ray. 4. Join up crosses with a ruler 5. Draw on a normal where the ray enters the glass block 6. Measure the angle of incidence and the angle of refraction and add these to your results table 7. Calculate the refractive 8. Repeat steps 2 – 7 using 9. Find an average of your
|
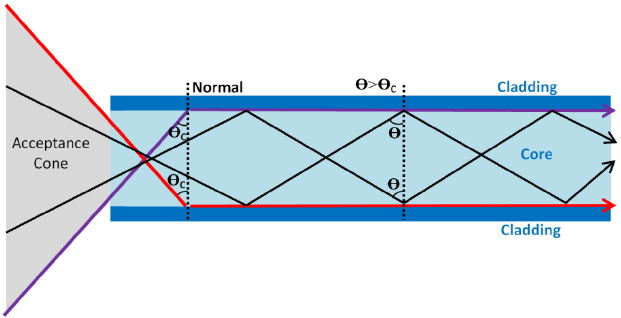
Total Internal Reflection:
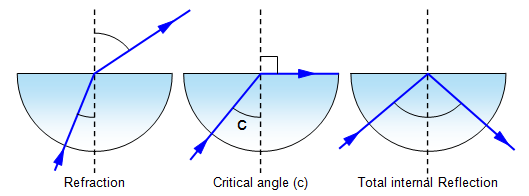
Critical Angle:
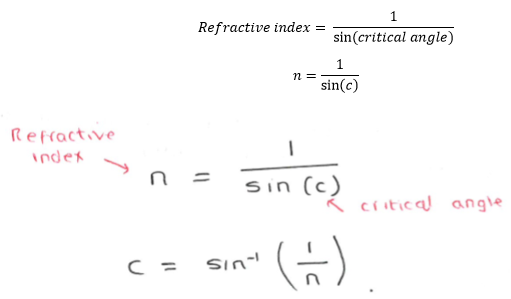
also remember:
critical angle = sin-1(1/n)
sound waves are longitudinal waves which can be reflected and refracted.
know the units for
Mass = kilogram (kg)
energy = joule (J)
velocity = metre/second (m/s)
acelleration = metre/ second 2 (m/s2)
force = newton (N)
time = second (s)
power = watt (W)
|
Energy Stores: Chemical – e.g. the food we eat Kinetic – movement energy Gravitational – objects that are lifted up Elastic – e.g. from springs Thermal – from hot objects Magnetic – objects in magnetic fields Electrostatic – charged objects Nuclear – stored within a nucleus
|
In any process energy is never created or destroyed. (It is just transferred from one store to another.)
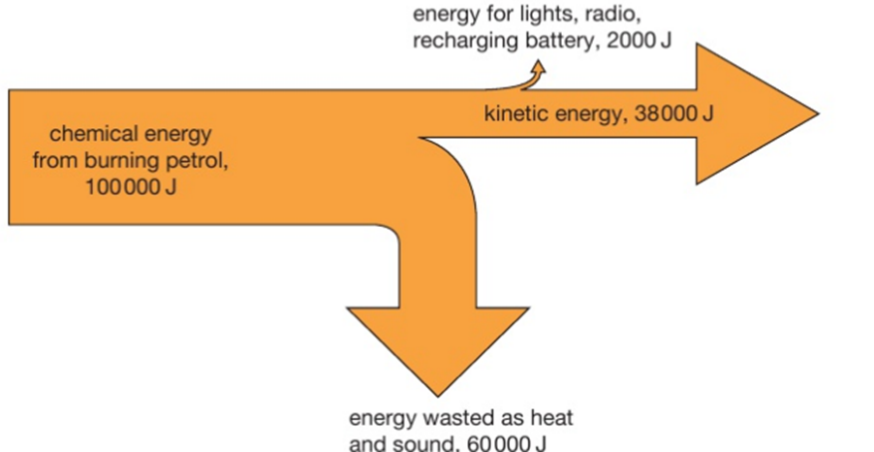
The energy flow is shown by arrows whose width is proportional to the amount of energy involved. The wasted and useful energy outputs are shown by different arrows.
Work Done (J) = Force (N) x distance moved (m)
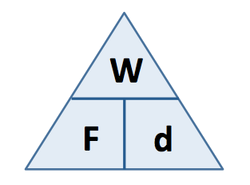
|
Work done = energy transferred |
Gravitational potential energy (J) = Mass (kg) x gravitational field strength (N/kg) x height (m)
Kinetic energy (J) = 0.5 x mass (kg) x velocity (m/s) 2
Because energy is conserved the decrease in GPE = increase in KE, for a falling object if no energy is lost to the surroundings
power is the rate of transfer of energy, or the rate of work done. so p = E/t
The units for:
temperature: degree Celsius (°C) or Kelvin (K)
Energy: Joule (J)
mass: Kilogram (kg)
density: kilogram/metre cubed (kg/m3)
distance: metre (m)
area: metre squared (m2)
volume: metre cubed (m3)
velocity: metre per second (m/s)
acceleration: metre per second squared (m/s2)
force: newton (N)
pressure: pascal (Pa)
the unit for
specific heat capacity: joules/kilogram degree Celsius (J/kg °C)
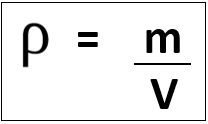
Units of density depend on units used for mass and volume:
E.g. mass in [g] and volume in [cm3] gives density in [g/cm3], however mass in [kg] and volume in [m3] gives density in [kg/m3].
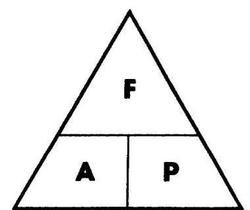
pressure (Pa) = force (N)/ area (m2)
Pressure difference [Pa] = Density [kg/m3] x g [N/kg] x Height [m]
ΔP = ρ g h
P1 – Patm = ρ g h
P1 = ρ g h + Patm
Gas laws:
Absolute zero:
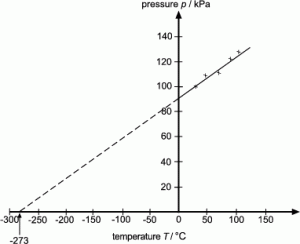
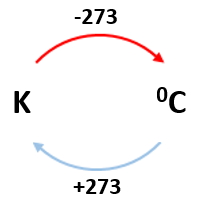
0 K = -273 0C
E.g. 100K = -1730C
2000C = 473K
As you increase the temperature of a gas, the kinetic energy of the gas particles increases and thus their average speed also increases.
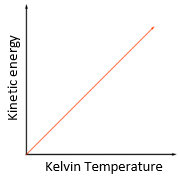
The Kelvin temperature of a gas is proportional to the average kinetic energy of its molecules.
P1/T1 = P2/T2
*Temperature must be in Kelvin
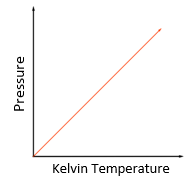
Temperature law:
For a fixed mass of gas at constant volume, the pressure is directly proportional to the Kelvin temperature
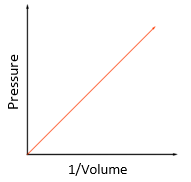
P1V1 = P2V2
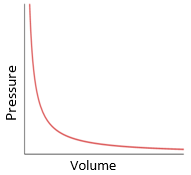
Boyle’s law:
For a fixed mass of gas at constant temperature, the pressure is inversely proportional to the volume.
The unit for:
Current : amps (A)
Potential Difference : volt (V)
power : watt (W)
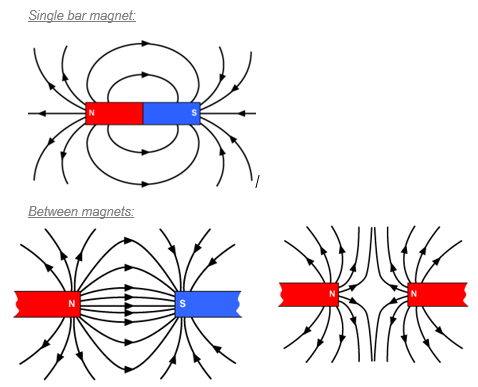
Opposites attract: North attracts South and South attracts North
Like charges repel: Two Norths will repel each other
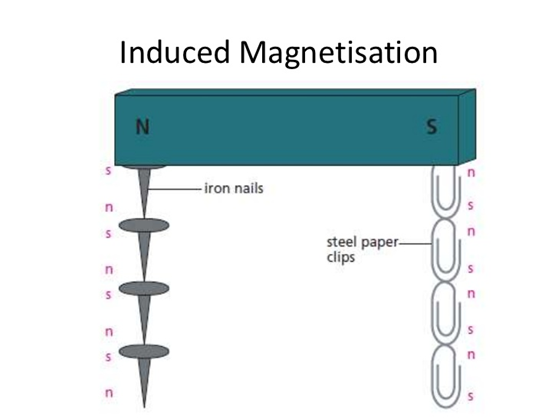
Permanent magnets are made of magnetically hard materials such as steel. These materials retain their magnetism once magnetised.
Some materials like iron are magnetically soft. They lose their magnetism once they are no longer exposed to a magnetic field. They are used as temporary magnets such as electromagnets.
Around every magnet there is a region of space where we can detect magnetism (where magnetic materials will be affected).
This is called the magnetic field and in a diagram we represent this with magnetic field lines.
The magnetic field lines should always point from north to south.
|
When magnetic materials are bought near or touch the pole of a strong or permanent magnet, they become magnets. This magnetic character is induced in the objects and it is removed when the permanent magnet is removed. This is a temporary magnet Magnetism is induced in the paperclips so each paperclip can attract another one |