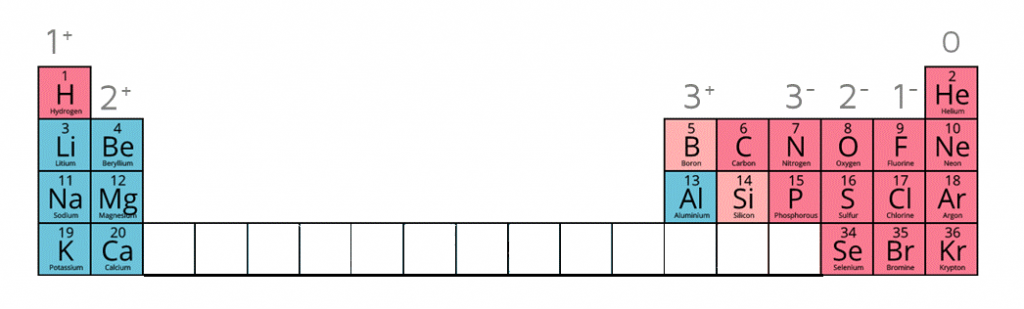
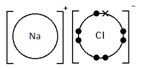
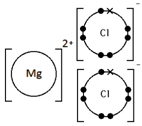
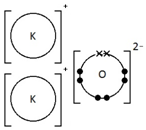
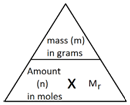
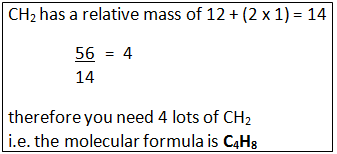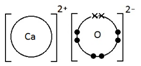Calculating the Empirical Formula
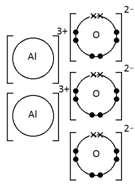
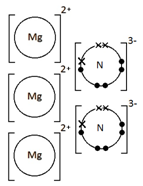
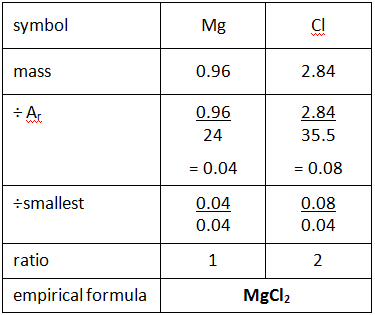 Example: What is the empirical formula of magnesium chloride if 0.96 g of magnesium combines with 2.84 g of chlorine?
Example: What is the empirical formula of magnesium chloride if 0.96 g of magnesium combines with 2.84 g of chlorine?
Step 1: Put the symbols for each element involved at the top of the page.
Step 2: Underneath, write down the masses of each element combining.
Step 3: Divide by their relative atomic mass (Ar).
Step 4: Divide all the numbers by the smallest of these numbers to give a whole number ratio.
Step 5: Use this to give the empirical formula.
(If your ratio is 1:1.5 then multiple each number by 2
If your ratio is 1:1.33 then x3. If your ratio is 1:1.25 x4)
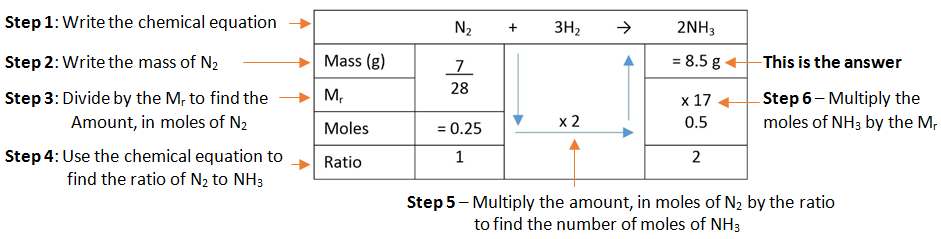
Calculating the Molecular Formula
If you know the empirical formula and the relative formula mass you can work out the molecular formula of a compound.
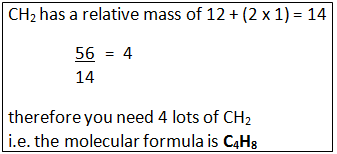
Example: A compound has the empirical formula CH2, and its relative formula mass is 56. Calculate the molecular formula.
Step 1: Calculate the relative mass of the empirical formula.
Step 2: Find out the number of times the relative mass of the empirical formula goes into the Mr of the compound.
Step 3: This tells how many times bigger the molecule formula is compared to the empirical formula.
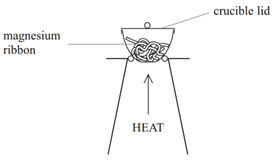
Empirical formula calculations involving water of crystallisation
When you heat a salt that contains water of crystallisation, the water is driven off leaving the anhydrous (without water) salt behind. For example, barium chloride crystals contain water of crystallisation, and therefore would have the formula BaCl2.nH2O where the symbol ‘n’ indicates the number of molecules of water of crystallisation. This value can be calculated using the following method.
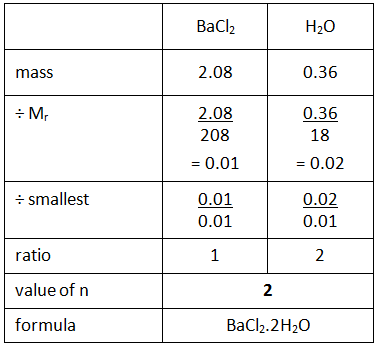
Example: If you heated hydrated barium chloride (BaCl2.nH2O) in a crucible you might end up with the following results.

Step 1: Calculate the mass of the anhydrous barium chloride (BaCl2) and the water (H2O) driven off.

Step 2: Use the empirical formula method to find the value of n in the formula.


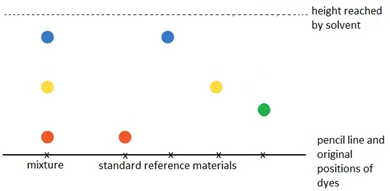
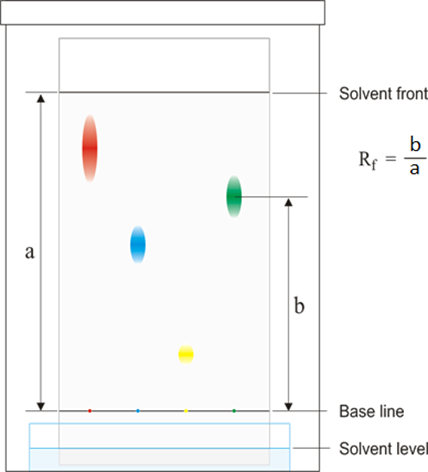
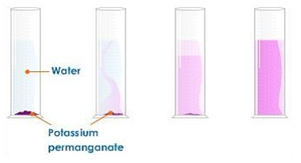 Dissolving potassium manganate(VII) in water demonstrates that the diffusion in liquids is very slow because there are only small gaps between the liquid particles into which other particles diffuse.
Dissolving potassium manganate(VII) in water demonstrates that the diffusion in liquids is very slow because there are only small gaps between the liquid particles into which other particles diffuse.
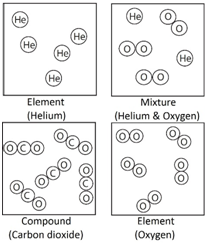
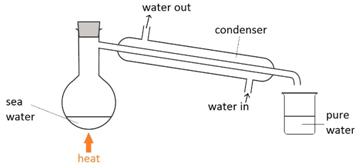
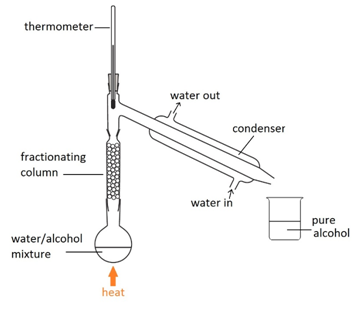
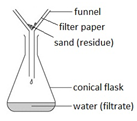 Filtration
Filtration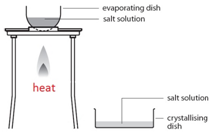 Gently heat the solution in an evaporating basin to evaporate some of the water
Gently heat the solution in an evaporating basin to evaporate some of the water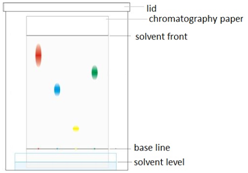
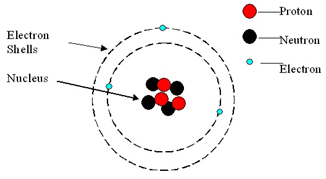

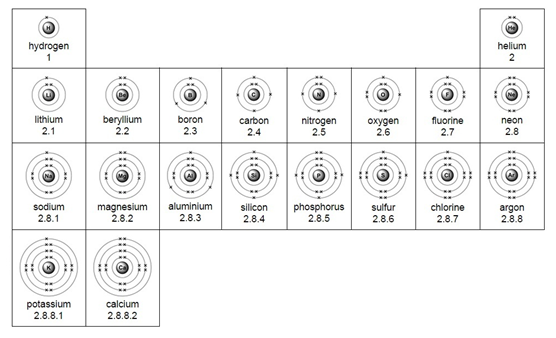
 Example: What is the empirical formula of magnesium chloride if 0.96 g of magnesium combines with 2.84 g of chlorine?
Example: What is the empirical formula of magnesium chloride if 0.96 g of magnesium combines with 2.84 g of chlorine?