4:34 (Triple only) know that carboxylic acids contain the functional group -COOH
Carboxylic acids contain the functional group -COOH
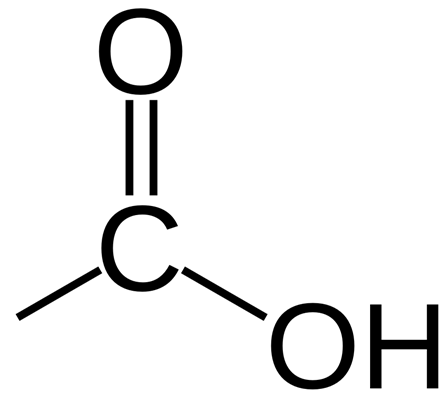
An example of a carboxylic acid is butanoic acid:
Carboxylic acids contain the functional group -COOH

An example of a carboxylic acid is butanoic acid:
The four simplest carboxylic acids are methanoic acid, ethanoic acid, propanoic acid and butanoic acid.
Methanoic acid
Displayed formula:
Molecular formula: CH₂O₂
Structural formula: HCOOH
Ethanoic acid
Displayed formula: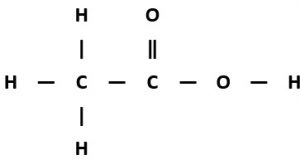
Molecular formula: C₂H₄O₂
Structural formula: CH₃COOH
Propanoic acid
Displayed formula:
Molecular formula: C₃H₆O₂
Structural formula: CH₃CH₂COOH
Butanoic acid
Displayed formula:
Molecular formula: C₄H₈O₂
Structural formula: CH₃CH₂CH₂COOH
This video introduces carboxylic acids:
Dilute carboxylic acids react with metals in the same way as other dilute acids (e.g. hydrochloric acid) only more slowly.
For example, dilute ethanoic acid reacts with magnesium with a lot of fizzing to produce a salt and hydrogen, leaving a colourless solution of magnesium ethanoate:
magnesium + ethanoic acid → magnesium ethanoate + hydrogen
Mg (s) + 2CH₃COOH (aq) → (CH₃COO)₂Mg (aq) + H₂ (g)
Dilute carboxylic acids react with metal carbonates as they do with other acids, to give a salt, carbon dioxide and water.
For example, dilute ethanoic acid reacts with sodium carbonate with a lot of fizzing to produce a salt, carbon dioxide and water, leaving a colourless solution of sodium ethanoate:
sodium carbonate + ethanoic acid → sodium ethanoate + carbon dioxide + water
Na₂CO₃ (s) + 2CH₃COOH (aq) → 2CH₃COONa (aq) + CO₂ (g) + H₂O (l)
As can be seen in the examples above the charge on the ethanoate ion is -1.
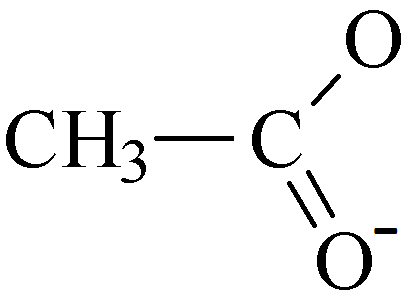
Vinegar is an aqueous solution containing ethanoic acid (CH₃COOH).
Esters contain the functional group -COO-
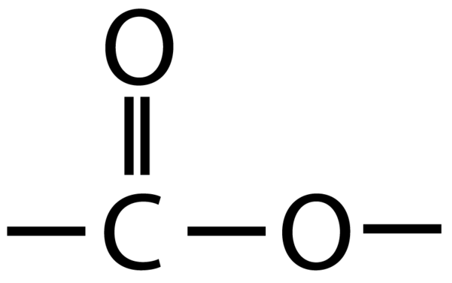
An example of an ester is ethyl ethanoate:
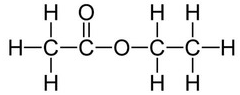
Ethyl ethanoate is the ester produced when ethanol and ethanoic acid react in the presence of an acid catalyst.
ethanoic acid + ethanol ⇋ ethyl ethanoate + water
CH₃COOH (l) + CH₃CH₂OH (l) ⇋ CH₃COOCH₂CH₃ (l) + H₂O (l)
The ethyl ethanoate produced is an ester.
The reaction is called esterification.
The reaction can also be described as a condensation reaction because water is made when two molecules join together.
Ethyl ethanoate is an ester.
Structural formula: CH₃COOCH₂CH₃
Displayed formula:
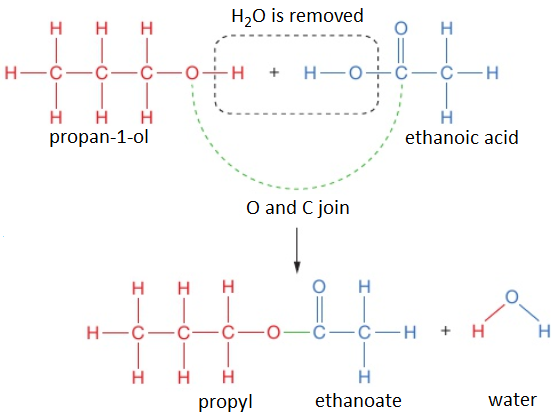
To work out the structure of the ester formed when an alcohol reacts with a carboxylic acid, it is easiest to first draw the structures of alcohol and acid and then remove the H₂O to see what is left when the molecules join.
For example, propan-1-ol and ethanoic acid react together to form propyl ethanoate and water.
propan-1-ol + ethanoic acid → propyl ethanoate + water
CH₃CH₂CH₂OH (l) + CH₃COOH (l) → CH₃COOCH₂CH₂CH₃ (l) + H₂O (l)
The displayed formula for esters is typically written to show the part which came from the carboxylic acid on the left:
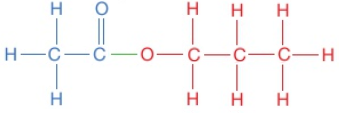
This is still called propyl ethanoate: the alcohol bit of the name (propyl) comes first and then the carboxylic acid bit (ethanoate), even though the displayed formula is typically written the other way around. Notice that the structural formulae for esters is also typically written with the carboxylic acid bit first, so propyl ethanoate is CH₃COOCH₂CH₂CH₃.
Esters are volatile compounds with distinctive smells. A volatile liquid is one that turns into to a vapour easily.
Esters are used as food flavourings and in perfumes.
Esters are often described as having a sweet, fruity smell. They typically smell of bananas, raspberries, pears or other fruit because esters occur in all these natural products.
Heating a mixture of ethanoic acid and ethanol produces a liquid called ethyl ethanoate. A few drops of concentrated sulfuric acid must be added for the reaction to work. The sulfuric acid acts as a catalyst.
ethanoic acid + ethanol ⇋ ethyl ethanoate + water
CH₃COOH (l) + CH₃CH₂OH (l) ⇋ CH₃COOCH₂CH₃ (l) + H₂O (l)
The ethyl ethanoate produced is an ester.
The reaction is called esterification.
The reaction can also be described as a condensation reaction because water is made when two molecules join together.
Notice that the reaction is reversible. Pure reactants are used to maximise the yield of ethyl ethanoate. Pure ethanoic acid is called glacial ethanoic acid.
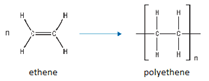
Monomers join together to form a long chain.
Polymer contains only single bonds.
To deduce the structure of the monomer from a repeat unit:
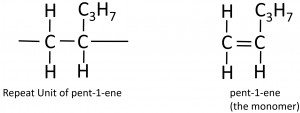
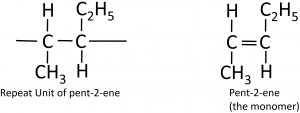
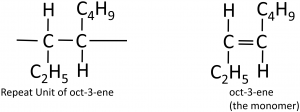
Here’s a more complicated example, going from the polymer to the structure of the monomer
This video introduces addition polymers:
[ Thanks to the amazing Ms B for this resource ]
Polymers are inert (unreactive) as they have strong C-C bonds.
This makes them non-biodegradeable.
Biodegradable: the breakdown of a substance by microorganisms.
if burnt the addition polymers could produce toxic gases such as carbon monoxide and hydrogen chloride.
Condensation polymers are formed by a condensation reaction.
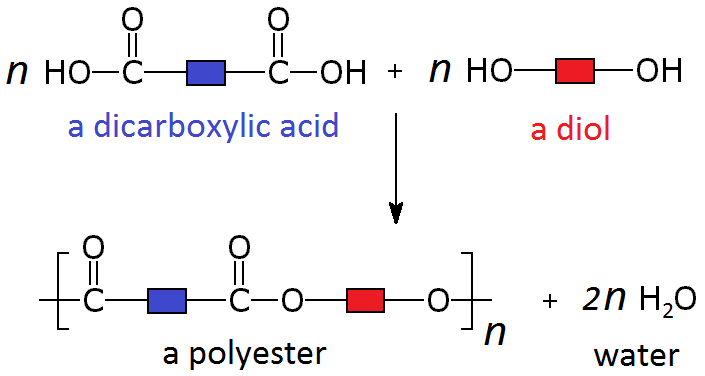
These polymers are formed by the combination of two different monomers, such as a dicarboxylic acid and a diol.
When these particular monomers join in an alternating pattern they form a long polymer called a polyester. Where each monomer joins to the next, a separate molecule of water is also produced.
Polyesters are polymers formed when two types of monomer join together alternately. Where each joins to the next a small molecule, such as water or hydrogen chloride, is lost. This is called a condensation polymerisation reaction.
One of the monomers is a diol, an alcohol with a -OH functional group at each end. An example is hexane-1,6-diol which has the structural formula CH₂OHCH₂CH₂CH₂CH₂CH₂OH and the displayed formula:
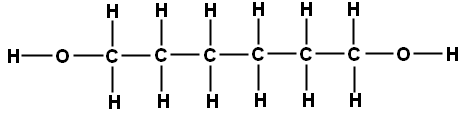
Since it is only the -OH functional groups which are important for polymerisation, this can we re-written with the central block of carbons represented as a block:
![]()
The other monomer is a dicarboxylic acid, a molecule with a -COOH functional group at each end. An example is hexane-1,6-dioic acid which has the structural formula HOOCCH₂CH₂CH₂CH₂COOH and the displayed formula:
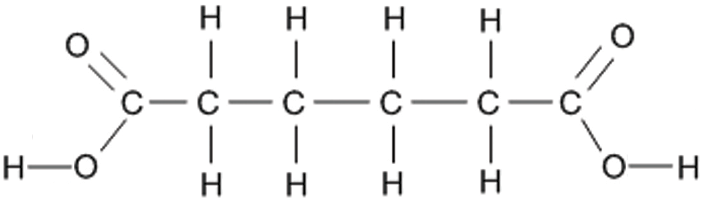
Since it is only the -COOH functional groups which are important for polymerisation, this can we re-written with the central block of 4 carbons represented as a block:
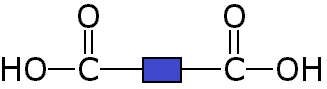
These two different types of monomer (the diol and the dicarboxylic acid) can join to form a polymer with the loss of a water molecule at every bond. As above, this can be simplified by only looking at the functional groups and representing the other carbons as blocks, so the whole process looks like:
A simple example of this is the condensation polymerisation reaction between ethanedioic acid and ethandiol:
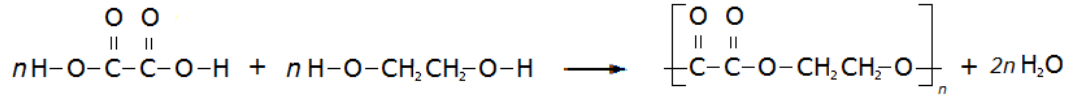
This video introduces the idea of condensation polymers:
There are environmental issues with the disposal of condensation polymers, though because of their ester linkage the issues are not as severe as with addition polymers. Normally condensation polymers can take hundreds of years to break down, but chemists has developed biopolyesters which break down much more quickly.
| Spec | Q'stion | Ans | MS |
|---|