Calculations practice – Molecular formulae
Refresh the page to get a different random selection of questions and answers.
Calculations practice – Reacting masses
Refresh the page to get a different random selection of questions and answers.
Calculations practice – Mixed calculations
Refresh the page to get a different random selection of questions and answers.
Calculate reacting masses from experimental data – QUIZ
[WpProQuiz 63]
[WpProQuiz 62]
[WpProQuiz 64]
[WpProQuiz 61]
[WpProQuiz 65]
[WpProQuiz 60]
Tricks for remembering the names of organic molecules
A good way to remember the names of organic molecules is to make up a silly mnemonic where the first letter of each word matches the first letter of the organic molecules. For example the first 10 alkanes in order are , Methane, Ethane, Propane, Butane, Pentane, Hexane, Heptane, Octane, Nonane and Decane. These can be memorised with “Many elephants prefer blue pinapples. However hungry orangutans never do.” This isn’t a very good mnemonic, but it is the one I made up for myself when I was at school, and you are best making up your own. The sillier, smellier and more colourful the better.
1:01 understand the three states of matter in terms of the arrangement, movement and energy of the particles
Solid
Arrangement: Particles are close together and regularly packed.
Movement: Particles vibrate around a fixed point.
Energy: Particles have less kinetic energy than both liquids and gasses.
Liquid

Arrangement: Particles are close together but irregular.
Movement: Particles are free to move.
Energy: Particles have less kinetic energy than gasses but more than solids.
Gas
Arrangement: Particles are far apart and there are no forces between them.
Movement: Particles are free to move.
Energy: Particles have more kinetic energy than liquids and solids.
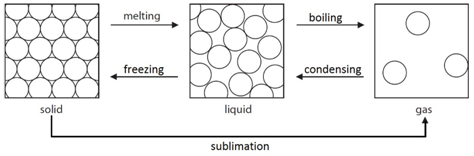
1:02 understand the interconversions between the three states of matter in terms of: the names of the interconversions, how they are achieved and the changes in arrangement, movement and energy of the particles
Melting: When a solid is heated, the energy makes the particles vibrate fast enough so that the forces of attraction between the particles break. For example H2O(s) –> H2O(l)
Freezing: When a liquid is cooled, the particles move slow enough so that the forces of attraction between them will hold them into a solid. For example H2O(l) –> H2O(s)
Boiling: When a liquid is heated strongly, the energy makes the particles move fast enough so that all forces of attraction are broken. For example H2O(l) –> H2O(g)
Condensing: When a gas is cooled, the particles move slow enough so that the forces of attraction between them will hold them as a liquid. For example H2O(g) –> H2O(l)
Sublimation: A small number of substances have the ability to change directly from a solid to a gas when heated. For example CO2(s) –> CO2(g)
1:03 understand how the results of experiments involving the dilution of coloured solutions and diffusion of gases can be explained
Diffusion is the spreading out of particles in a gas or liquid. There is a net movement of particles from areas of high concentration to areas of low concentration until a uniform concentration is achieved.
i) dilution of coloured solutions
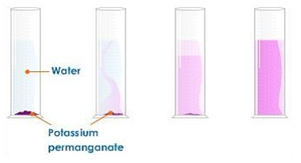 Dissolving potassium manganate(VII) in water demonstrates that the diffusion in liquids is very slow because there are only small gaps between the liquid particles into which other particles diffuse.
Dissolving potassium manganate(VII) in water demonstrates that the diffusion in liquids is very slow because there are only small gaps between the liquid particles into which other particles diffuse.
The random motion of particles cause the purple colour to eventually be evenly spread out throughout the water.
Adding more water to the solution causes the potassium manganate(VII) particles to spread out further apart therefore the solutions becomes less purple. This is called dilution.
ii) diffusion experiments
When ammonia gas and hydrogen chloride gas mix, they react together to form a white solid called ammonium chloride.
ammonia + hydrogen chloride –> ammonium chloride
NH3(g) + HCl(g) –> NH4Cl(s)
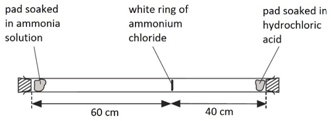
A cotton wool pad was soaked in ammonia solution and another was soaked in hydrogen chloride solution. The two pads were then put into opposite ends of a dry glass tube at the same time.
The white ring of ammonium chloride forms closer to the hydrochloric acid end because ammonia particles are lighter than hydrogen chloride particles and therefore travel faster.
Even though these particles travel at several hundred metres per second, it takes about 5 min for the ring to form. This is because the particles move in random directions and will collide with air particles in the tube.
Diffusion – video
This video is a great way to see the diffusion of particles in action:
1:04 know what is meant by the terms: solvent, solute, solution, saturated solution
When a solid dissolves in a liquid:
- the substance that dissolves is called the solute
- the liquid in which it dissolves is called the solvent
- the liquid formed is a solution
- a saturated solution is a solution into which no more solute can be dissolved
1:05 (Triple only) know what is meant by the term solubility in the units g per 100g of solvent
Solubility is defined in terms of the maximum mass of a solute that dissolves in 100g of solvent. The mass depends on the temperature.
For example, the solubility of sodium chloride (NaCl) in water at 25⁰C is about 36g per 100g of water.
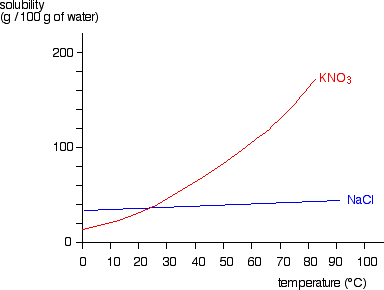
1:06 (Triple only) understand how to plot and interpret solubility curves
The solubility of solids changes as temperature changes. This can be plotted on a solubility curve.
The salts shown on this graph are typical: the solubility increases as temperature increases.
For example, the graph above shows that in 100g of water at 50⁰C the maximum mass of potassium nitrate (KNO₃) which will dissolve is 80g.
However, if the temperature were 80⁰C a mass of 160g of potassium nitrate (KNO₃) would dissolve in 100g of water.
 Simple molecules & covalent bonding flashcards
Simple molecules & covalent bonding flashcards