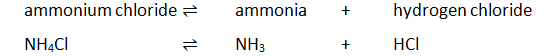The rate of a chemical reaction can be measured either by how quickly reactants are used up or how quickly the products are formed.
The rate of reaction can be calculated using the following equation:

The units for rate of reaction will usually be grams per min (g/min)
An investigation of the reaction between marble chips and hydrochloric acid:

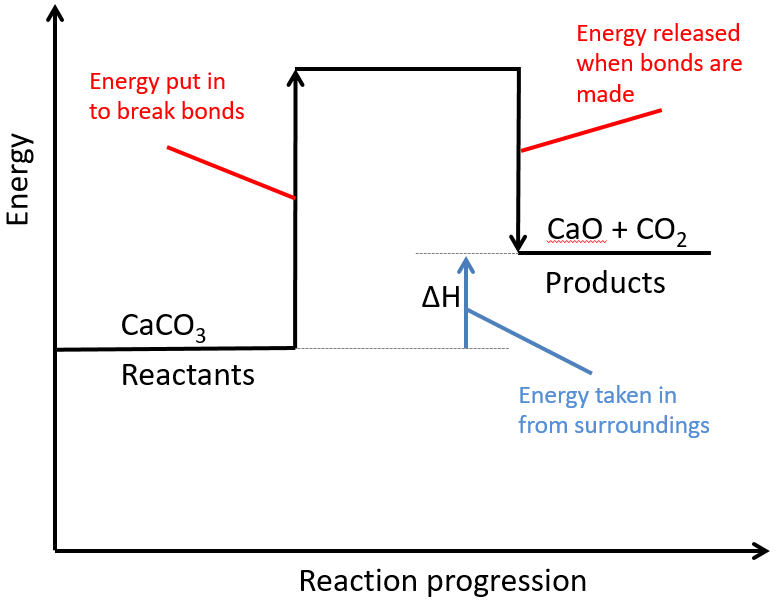
 Marble chips, calcium carbonate (CaCO3) react with hydrochloric acid (HCl) to produce carbon dioxide gas. Calcium chloride solution is also formed.
Marble chips, calcium carbonate (CaCO3) react with hydrochloric acid (HCl) to produce carbon dioxide gas. Calcium chloride solution is also formed.
Using the apparatus shown the change in mass of carbon dioxide can be measure with time.
As the marble chips react with the acid, carbon dioxide is given off.
The purpose of the cotton wool is to allow carbon dioxide to escape, but to stop any acid from spraying out.
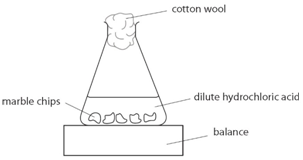
The mass of carbon dioxide lost is measured at intervals, and a graph is plotted:
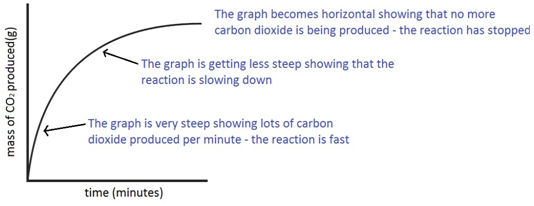
Experiment to investigate the effects of changes in surface area of solid on the rate of a reaction:
The experiment is repeated using the same mass of chips, but this time the chips are larger, i.e. have a smaller surface area.
Since the surface area is smaller, the rate of reaction is less.
Both sets of results are plotted on the same graph.
If instead the chips were smashed into powder (and again same mass of chips used) the surface area would be much larger and so the rate of reaction higher (steeper line on graph).
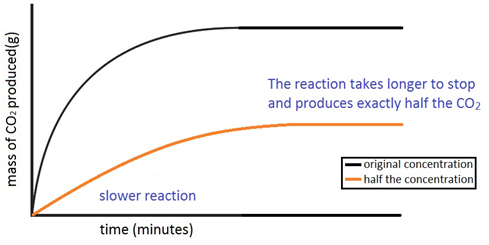
Experiment to investigate the effects of changes in concentration of solutions on the rate of a reaction:
The experiment is again repeated using the exact same quantities of everything but this time with half the concentration of acid. The marble chips must however be in excess. The reaction with the half the concentration of acid happens slower and produces half the amount of carbon dioxide.
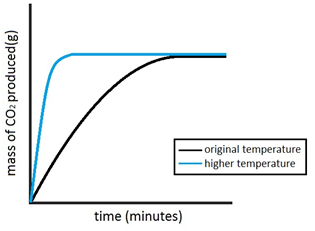
Experiment to investigate the effects of changes in temperature on the rate of a reaction:
The experiment is once again repeated using the exact same quantities of everything but this time at a higher temperature. The reaction with the higher temperature happens faster.
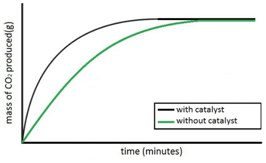
Experiment to investigate the effects of the use of a catalyst on the rate of a reaction:
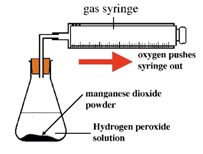
Hydrogen peroxide naturally decomposes slowly producing water and oxygen gas.
Manganese (IV) oxide can be used as a catalyst to speed up the rate of reaction.

The rate of reaction can be measured by measuring the volume of oxygen produced at regular intervals using a gas syringe.
Both sets of results are plotted on the same graph.
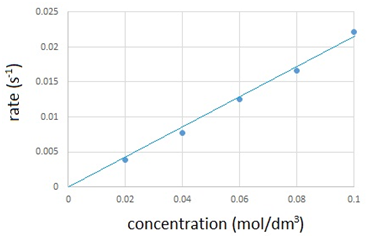
Experiment to investigate the reaction between varying concentrations of sodium thiosulfate and hydrochloric acid
Sodium thiosulfate (Na2S2O3) and hydrochloric acid (HCl) are both colourless solutions. They react to form a yellow precipitate of sulfur.
sodium thiosulfate + hydrochloric acid → sodium chloride + sulfur dioxide + sulfur + water
Na2S2O3(aq) + 2HCl(aq) → 2NaCl(aq) + SO2(g) + S(s) + H2O(l)
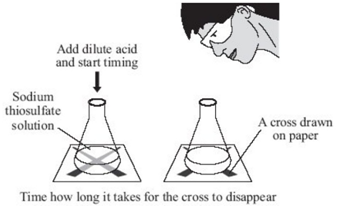
To investigate the effects of changes in concentration of sodium thiosulfate on the rate of a reaction, the conical flask is placed above a cross. The reaction mixture is observed from directly above and the time for a cross to disappear is measured. The cross disappears because a precipitate of sulfur is formed.
In order to change the concentration of sodium thiosulfate, the volumes of sodium thiosulfate and water are varied (see results table). However the total volume of solution must always be kept the same as to ensure that the depth of the solution remains constant.
In this reaction, sulfur dioxide gas (SO2), which is poisonous is produced therefore the experiment must be carried out in a well ventilated room.
The results are recorded in the table below and then plotted onto a graph.
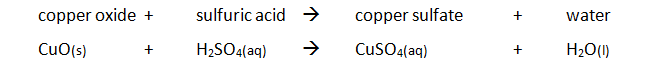
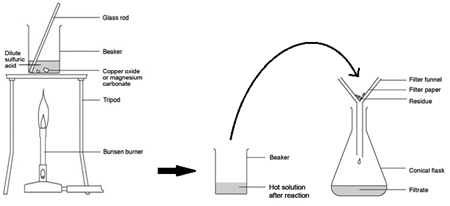


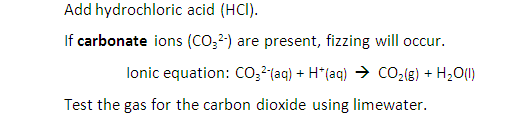


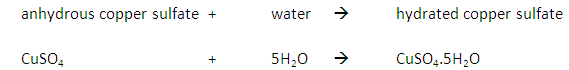
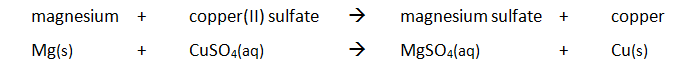
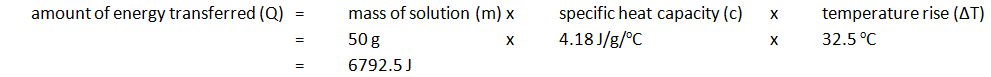
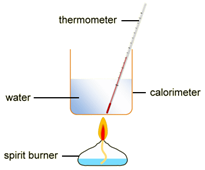
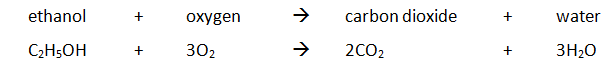
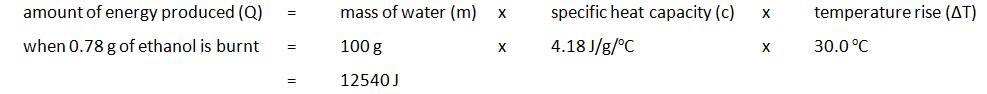

 Marble chips, calcium carbonate (CaCO3) react with hydrochloric acid (HCl) to produce carbon dioxide gas. Calcium chloride solution is also formed.
Marble chips, calcium carbonate (CaCO3) react with hydrochloric acid (HCl) to produce carbon dioxide gas. Calcium chloride solution is also formed.






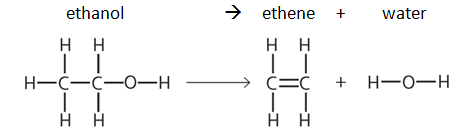
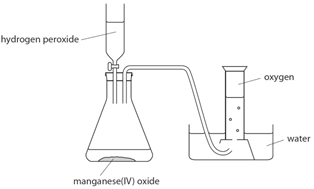 Oxygen (O2) is made in the lab from hydrogen peroxide (H2O2) using manganese(IV) oxide (MnO2) as a catalyst.
Oxygen (O2) is made in the lab from hydrogen peroxide (H2O2) using manganese(IV) oxide (MnO2) as a catalyst.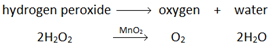
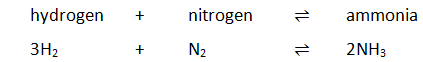
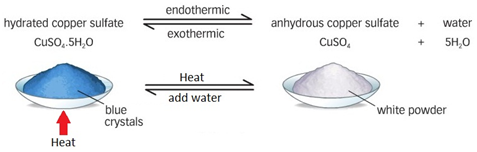
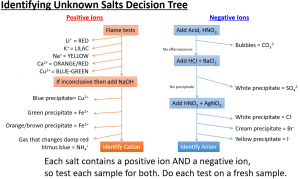
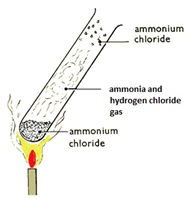 Heating ammonium chloride
Heating ammonium chloride