4:11 know that a fuel is a substance that, when burned, releases heat energy
A fuel is a substance that, when burned, releases heat energy (exothermic reaction).
A fuel is a substance that, when burned, releases heat energy (exothermic reaction).
Complete Combustion happens when there is enough oxygen available, producing carbon dioxide (CO2) and water (H2O)
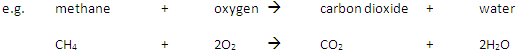
Incomplete Combustion happens when there is not enough oxygen available, with possible products being carbon monoxide (CO), carbon (C, soot), carbon dioxide (CO2) and water (H2O)
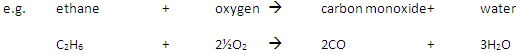
Carbon monoxide may be produced from the incomplete combustion of fuels:
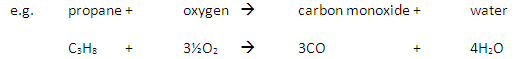
Carbon monoxide is poisonous because it reduces the capacity of the blood to carry oxygen.
When fuels are burned in vehicle engines, high temperatures are reached.
At these high temperatures nitrogen and oxygen from the air react to produce nitrogen oxides:
nitrogen + oxygen → nitrogen oxides
eg
N2 (g) + O2 (g) → 2NO (g)
In the atmosphere these nitrogen oxides can combine with water to produce nitric acid (HNO3).
Fossil fuels such as coal, gas and oil are derived from crude oil.
These fuels are hydrocarbons, but also include impurities such as sulfur.
When the fuels are burned, sulfur dioxide is produced which can escape into the atmosphere:
S (s) + O₂ (g) → SO₂ (g)
Acids formed in the atmosphere can fall as acid rain. This can be a major problem, killing trees and fish in lakes. The acid rain also corrodes limestone buildings and marble statues since these are both made of calcium carbonate (CaCO₃). Some metals such as iron are also attacked by acid rain.
Sulfur dioxide released into the atmosphere from the burning of fossil fuels can react with water and oxygen to make sulfuric acid (H₂SO₄):
2SO₂ (g) + 2H₂O (l) + O₂ (g) → 2H₂SO₄ (aq)
Also, if sulfur dioxide in the atmosphere reacts with just water, a weaker acid called sulfurous acid (H₂SO₃) is formed:
SO₂ (g) + H₂O (l) → H₂SO₃ (aq)
In car engines the temperature is high enough for the nitrogen in the air to react with oxygen to produce oxides of nitrogen, e.g:
N₂ (g) + O₂ (g) → NO₂ (g)
In the atmosphere these nitrogen oxides can produce nitric acid (HNO₃).
Cracking involves the thermal decomposition of long-chain alkanes into shorter-chain alkanes and alkenes:
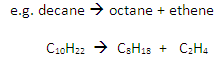
Conditions
Temperature: 600oC
Catalyst: aluminium oxide, Al2O3
Cracking converts long chain hydrocarbons into short chain hydrocarbons.
Long-chain alkanes are broken down into alkanes and alkenes of shorter length.
Crude oil contains a surplus long chains.
Shorter chain hydrocarbons are in greater demand, e.g. petrol.
Cracking also produces alkenes which are used in making polymers and ethanol.
Alkanes have the general formula CnH2n+2
This means that to work out the number of hydrogens, you double the number of carbons and then add two.
Saturated: A molecule containing only single bonds between carbon atoms. For example, alkanes as described as saturated molecules.
Unsaturated: A molecule containing a carbon-carbon double or triple bond. For example, alkenes as described as unsaturated molecules.
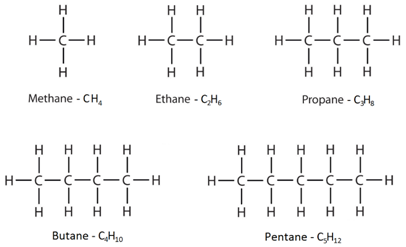
The displayed formulae show all the atoms and bonds drawn out.
The molecular formulae just show the number of each type of atom in the molecule.
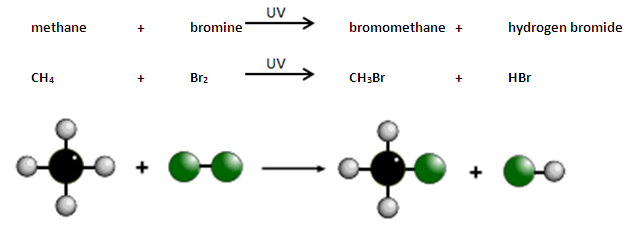
Alkanes react with bromine in the presence of UV light, e.g. sunlight.
A hydrogen atom in the alkane is replaced by a bromine atom.
This is known as substitution.
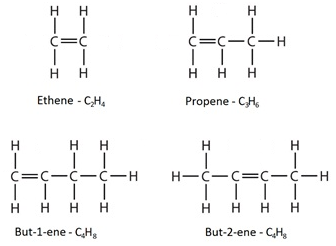
Alkenes are a homologous series of hydrocarbons which contain a carbon-carbon double bond. This double bond is shown in formulae as a double line.
The names of alkenes end with “ene”.
An example is ethene, the structural formula for which is CH₂ = CH₂
For a molecule with more than two carbon atoms, the position of the double bond within the molecule can vary as indicated by the name and the structural formula.
Alkenes have the general formula CnH2n
So an alkene always has twice as many hydrogen atoms as carbon atoms.
Saturated: A molecule containing only single bonds between carbon atoms. For example, alkanes as described as saturated molecules.
Unsaturated: A molecule containing a carbon-carbon double or triple bond. For example, alkenes as described as unsaturated molecules.
The displayed formulae show all the atoms and bonds drawn out.
The molecular formulae just show the number of each type of atom in the molecule.
Alkenes react with bromine water. UV light is not required for this reaction.
The double bond is broken and the bromine atoms are added. This is an addition reaction.
During this reaction there is a colour change from orange to colourless.
For example:
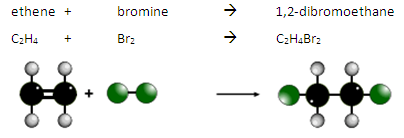
This is how we can test for the presence of an alkene or another type of unsaturated molecule.
In the absence of UV light an alkane added to bromine water will not react: the bromine water will stay orange.
However, alkenes react with bromine water even without UV light. There will be a colour change of orange to colourless.
The member of the homologous series called Alcohols have names which end in “ol”. Examples are methanol, ethanol and propanol.
Alcohols all contain an -OH functional group attached to a hydrocarbon chain.
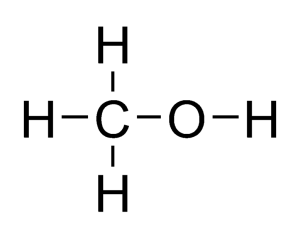
Structural formula and displayed formula for methanol:
CH₃-OH
Structural formula and displayed formula for ethanol:
CH₃-CH₂-OH (or simply C₂H₅OH)
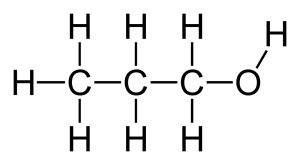
Structural formula and displayed formula for propan-1-ol:
CH₃-CH₂-CH₂-OH
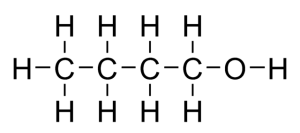
Structural formula and displayed formula for butan-1-ol:
CH₃-CH₂-CH₂-CH₂-OH
1) Ethanol can be oxidised by complete combustion. With excess oxygen the complete combustion of ethanol (C₂H₅OH) in air produces carbon dioxide and water:
C₂H₅OH (l) + 3O₂ (g) → 2CO₂ (g) + 3H₂O (l)
2) Ethanol can be oxidised in air in the presence of microorganisms (‘microbial oxidation’) to form ethanoic acid (CH₃COOH).
3) Ethanol can be oxidised by heating with the oxidising agent potassium dichromate(VI) (K₂Cr₂O₇) in dilute sulfuric acid (H₂SO₄).
In the equation below, [O] means oxygen from an oxidising agent.
CH₃CH₂OH + 2[O] → CH₃COOH + H₂O
This mixture starts orange but when the reaction happens turns green which indicates the presence of Cr³⁺ ions which are formed when the potassium dichromate(VI) is reduced.
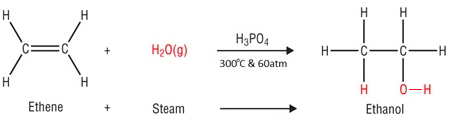 In the hydration of ethene, ethanol is made by passing ethene and steam over a catalyst.
In the hydration of ethene, ethanol is made by passing ethene and steam over a catalyst.
Water is added to ethene, this is known as hydration.
Conditions
Catalyst: Phosphoric acid (H3PO4)
Temperature: 300°C
Pressure: 60 atm
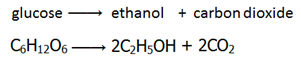 Fermentation is the conversion of sugar, e.g. glucose into ethanol by enzymes from yeast.
Fermentation is the conversion of sugar, e.g. glucose into ethanol by enzymes from yeast.
Conditions
Catalyst: Zymase (enzyme found in yeast)
Temperature: 30°C – The process is carried out at low temperatures as not to denature the enzymes.
Other: Anaerobic (no oxygen present) – if oxygen were present, the yeast produce carbon dioxide and water instead of ethanol.
In the production of ethanol the process of fermentation is carried out at a low temperature (30⁰-40⁰).
Above 40⁰ the enzymes would permanently lose their structure (denature).
At a temperature lower than 30⁰ the process would be too slow.
Fermentation is conducted in the absence of air. In the presence of air (aerobic conditions), enzymes in the yeast produce carbon dioxide and water instead of ethanol.
Also, in the presence of air, the ethanol can oxidise to ethanoic acid.
Carboxylic acids contain the functional group -COOH
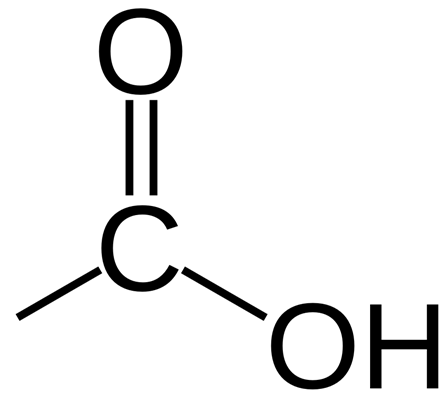
An example of a carboxylic acid is butanoic acid:
The four simplest carboxylic acids are methanoic acid, ethanoic acid, propanoic acid and butanoic acid.
Methanoic acid
Displayed formula:
Molecular formula: CH₂O₂
Structural formula: HCOOH
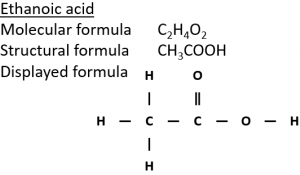
Ethanoic acid
Displayed formula: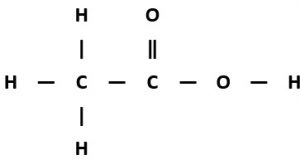
Molecular formula: C₂H₄O₂
Structural formula: CH₃COOH
Propanoic acid
Displayed formula:
Molecular formula: C₃H₆O₂
Structural formula: CH₃CH₂COOH
Butanoic acid
Displayed formula:
Molecular formula: C₄H₈O₂
Structural formula: CH₃CH₂CH₂COOH
Dilute carboxylic acids react with metals in the same way as other dilute acids (e.g. hydrochloric acid) only more slowly.
For example, dilute ethanoic acid reacts with magnesium with a lot of fizzing to produce a salt and hydrogen, leaving a colourless solution of magnesium ethanoate:
magnesium + ethanoic acid → magnesium ethanoate + hydrogen
Mg (s) + 2CH₃COOH (aq) → (CH₃COO)₂Mg (aq) + H₂ (g)
Dilute carboxylic acids react with metal carbonates as they do with other acids, to give a salt, carbon dioxide and water.
For example, dilute ethanoic acid reacts with sodium carbonate with a lot of fizzing to produce a salt, carbon dioxide and water, leaving a colourless solution of sodium ethanoate:
sodium carbonate + ethanoic acid → sodium ethanoate + carbon dioxide + water
Na₂CO₃ (s) + 2CH₃COOH (aq) → 2CH₃COONa (aq) + CO₂ (g) + H₂O (l)
As can be seen in the examples above the charge on the ethanoate ion is -1.
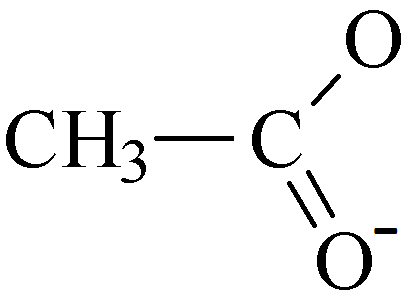
Vinegar is an aqueous solution containing ethanoic acid (CH₃COOH).
Esters contain the functional group -COO-
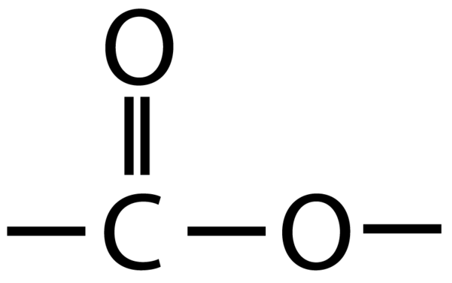
An example of an ester is ethyl ethanoate:
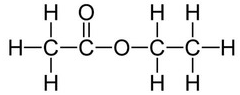
Ethyl ethanoate is the ester produced when ethanol and ethanoic acid react in the presence of an acid catalyst.
ethanoic acid + ethanol ⇋ ethyl ethanoate + water
CH₃COOH (l) + CH₃CH₂OH (l) ⇋ CH₃COOCH₂CH₃ (l) + H₂O (l)
The ethyl ethanoate produced is an ester.
The reaction is called esterification.
The reaction can also be described as a condensation reaction because water is made when two molecules join together.
Ethyl ethanoate is an ester.
Structural formula: CH₃COOCH₂CH₃
Displayed formula:
To work out the structure of the ester formed when an alcohol reacts with a carboxylic acid, it is easiest to first draw the structures of alcohol and acid and then remove the H₂O to see what is left when the molecules join.
For example, propan-1-ol and ethanoic acid react together to form propyl ethanoate and water.
propan-1-ol + ethanoic acid → propyl ethanoate + water
CH₃CH₂CH₂OH (l) + CH₃COOH (l) → CH₃COOCH₂CH₂CH₃ (l) + H₂O (l)
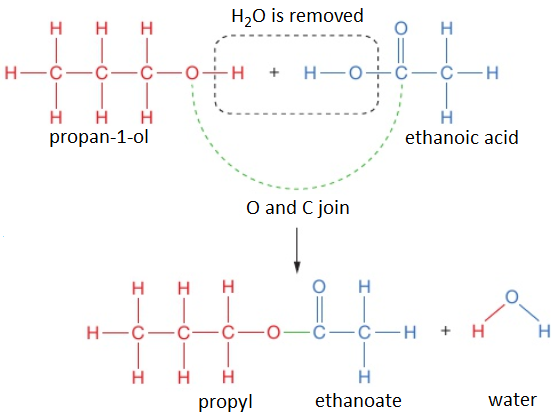
The displayed formula for esters is typically written to show the part which came from the carboxylic acid on the left:
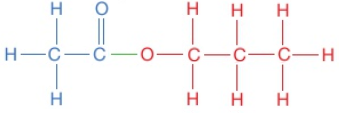
This is still called propyl ethanoate: the alcohol bit of the name (propyl) comes first and then the carboxylic acid bit (ethanoate), even though the displayed formula is typically written the other way around. Notice that the structural formulae for esters is also typically written with the carboxylic acid bit first, so propyl ethanoate is CH₃COOCH₂CH₂CH₃.
Esters are volatile compounds with distinctive smells. A volatile liquid is one that turns into to a vapour easily.
Esters are used as food flavourings and in perfumes.
Esters are often described as having a sweet, fruity smell. They typically smell of bananas, raspberries, pears or other fruit because esters occur in all these natural products.
Heating a mixture of ethanoic acid and ethanol produces a liquid called ethyl ethanoate. A few drops of concentrated sulfuric acid must be added for the reaction to work. The sulfuric acid acts as a catalyst.
ethanoic acid + ethanol ⇋ ethyl ethanoate + water
CH₃COOH (l) + CH₃CH₂OH (l) ⇋ CH₃COOCH₂CH₃ (l) + H₂O (l)
The ethyl ethanoate produced is an ester.
The reaction is called esterification.
The reaction can also be described as a condensation reaction because water is made when two molecules join together.
Notice that the reaction is reversible. Pure reactants are used to maximise the yield of ethyl ethanoate. Pure ethanoic acid is called glacial ethanoic acid.
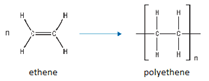
Monomers join together to form a long chain.
Polymer contains only single bonds.
To deduce the structure of the monomer from a repeat unit:
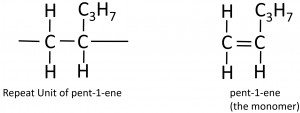
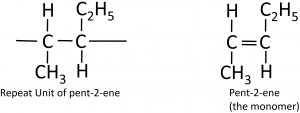
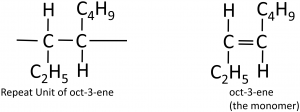
Here’s a more complicated example, going from the polymer to the structure of the monomer
Polymers are inert (unreactive) as they have strong C-C bonds.
This makes them non-biodegradeable.
Biodegradable: the breakdown of a substance by microorganisms.
if burnt the addition polymers could produce toxic gases such as carbon monoxide and hydrogen chloride.
Condensation polymers are formed by a condensation reaction.
These polymers are formed by the combination of two different monomers, such as a dicarboxylic acid and a diol.
When these particular monomers join in an alternating pattern they form a long polymer called a polyester. Where each monomer joins to the next, a separate molecule of water is also produced.
Polyesters are polymers formed when two types of monomer join together alternately. Where each joins to the next a small molecule, such as water or hydrogen chloride, is lost. This is called a condensation polymerisation reaction.
One of the monomers is a diol, an alcohol with a -OH functional group at each end. An example is hexane-1,6-diol which has the structural formula CH₂OHCH₂CH₂CH₂CH₂CH₂OH and the displayed formula:
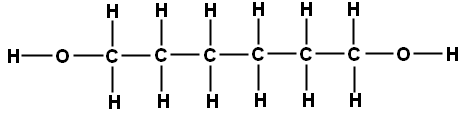
Since it is only the -OH functional groups which are important for polymerisation, this can we re-written with the central block of carbons represented as a block:
![]()
The other monomer is a dicarboxylic acid, a molecule with a -COOH functional group at each end. An example is hexane-1,6-dioic acid which has the structural formula HOOCCH₂CH₂CH₂CH₂COOH and the displayed formula:
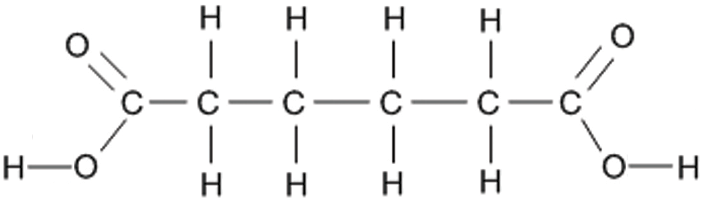
Since it is only the -COOH functional groups which are important for polymerisation, this can we re-written with the central block of 4 carbons represented as a block:
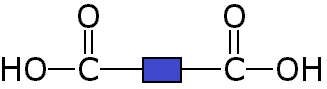
These two different types of monomer (the diol and the dicarboxylic acid) can join to form a polymer with the loss of a water molecule at every bond. As above, this can be simplified by only looking at the functional groups and representing the other carbons as blocks, so the whole process looks like:
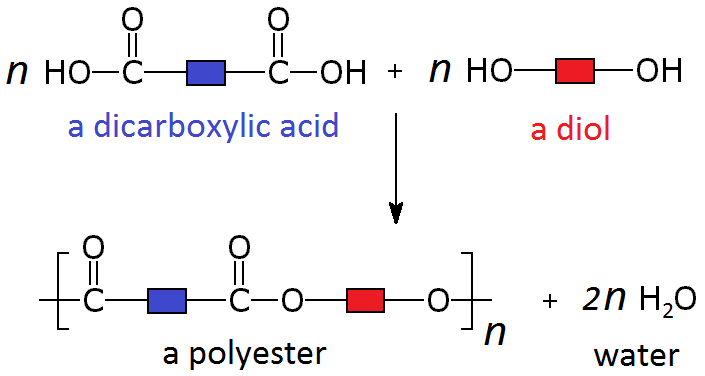
A simple example of this is the condensation polymerisation reaction between ethanedioic acid and ethandiol:
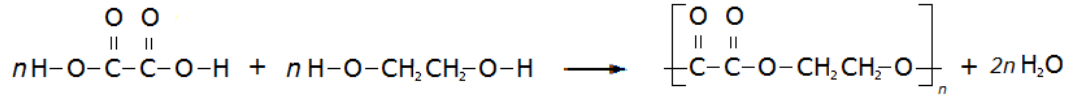
There are environmental issues with the disposal of condensation polymers, though because of their ester linkage the issues are not as severe as with addition polymers. Normally condensation polymers can take hundreds of years to break down, but chemists has developed biopolyesters which break down much more quickly.