2.18 know that the voltage across two components connected in parallel is the same
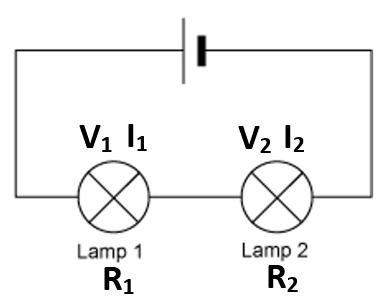
VT = V1 = V2
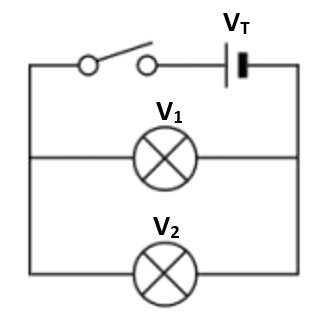
VT = V1 + V2
IT = I1 = I2
RT = R1 + R2
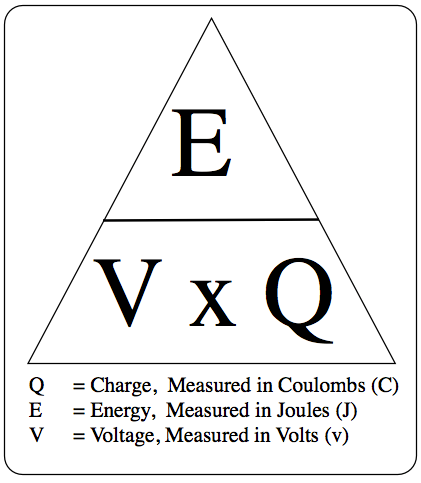
Energy Transferred (J) = charge (C) x Voltage (V)
Conducting Materials:
Will conduct electricity
Insulating Materials:
Will not conduct electricity
the units for:
angle = degree (°)
frequency = hertz (Hz)
wavelength = metre (m)
velocity = metre/second (m/s)
time = second (s)
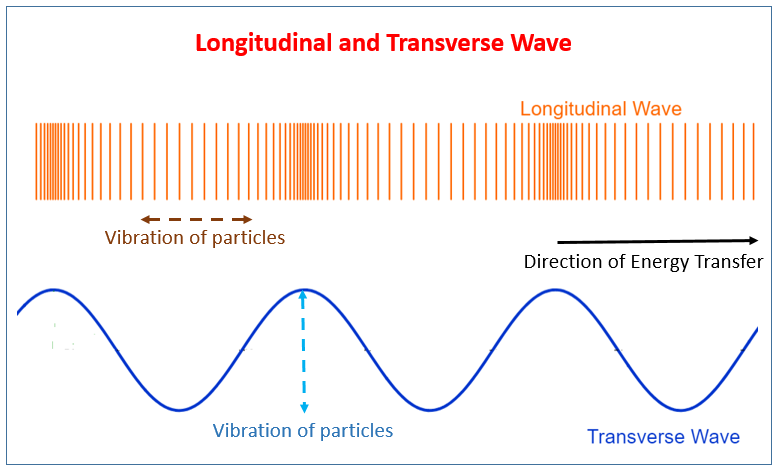
Transverse Waves:
Longitudinal Waves:
Key Definitions:
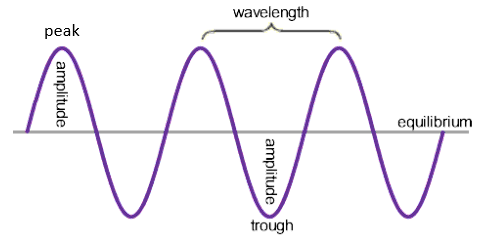
Waves can transfer energy and information with out transferring matter, for example sun light, it transfers energy as it makes the earth warm without bringing any matter.
wave speed (m/s) = frequency (Hz) x Wavelength (m)
Frequency (Hz) = 1/ Time Period (s)
You will need to use any of the in 3.05 and 3.06 to solve problems to do with sound waves and electromagnetic (light) waves
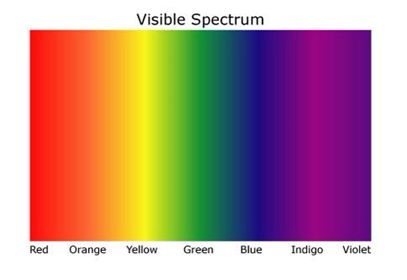
Electromagnetic Spectrum:
Radio Waves
Microwaves
Infrared (IR)
Visible Light
Ultraviolet (UV)
X – Rays
Gamma Rays
these are written in order of increasing frequency, lowest at the top
and decreasing wavelength, lowest at the bottom.
the colours displayed are in order of lowest frequency to the left highest frequency to the right.
|
uses of electromagnetic radiations, including: |
the detrimental effects of excessive exposure of the human body to electromagnetic waves:
• microwaves: internal heating of body tissue
• infrared: skin burns
• ultraviolet: damage to surface cells and blindness
• gamma rays: cancer, mutation
to reduce the risks:
light is a transverse wave that can be reflected and refracted
|
1. Set up your apparatus as shown in the diagram using a rectangular block. 2. Shine the light ray through the glass block 3. Use crosses to mark the path of the ray. 4. Join up crosses with a ruler 5. Draw on a normal where the ray enters the glass block 6. Measure the angle of incidence and the angle of refraction and add these to your results table 7. Comment on how the speed of the light has changed as the light moves between the mediums. 8. Repeat this for different angles of incidence and different glass prisms. |
|
1. Set up your apparatus as shown in the diagram using a rectangular block. 2. Shine the light ray through the glass block 3. Use crosses to mark the path of the ray. 4. Join up crosses with a ruler 5. Draw on a normal where the ray enters the glass block 6. Measure the angle of incidence and the angle of refraction and add these to your results table 7. Calculate the refractive 8. Repeat steps 2 – 7 using 9. Find an average of your
|
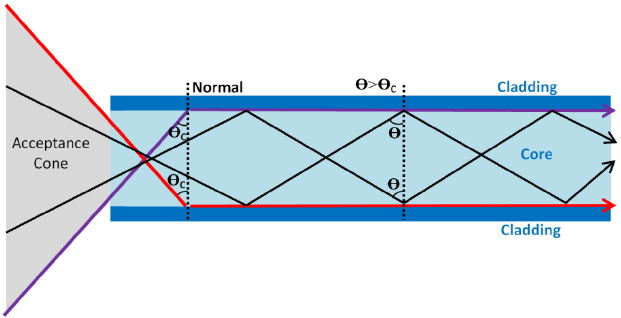
Total Internal Reflection:
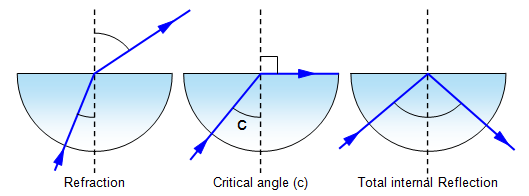
Critical Angle:
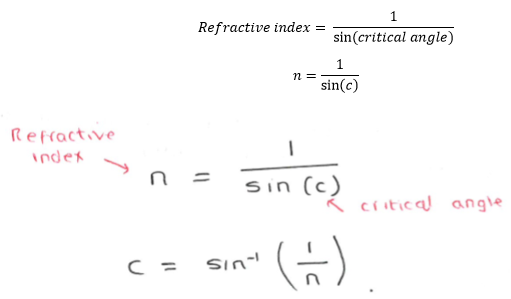
also remember:
critical angle = sin-1(1/n)
sound waves are longitudinal waves which can be reflected and refracted.
know the units for
Mass = kilogram (kg)
energy = joule (J)
velocity = metre/second (m/s)
acelleration = metre/ second 2 (m/s2)
force = newton (N)
time = second (s)
power = watt (W)
|
Energy Stores: Chemical – e.g. the food we eat Kinetic – movement energy Gravitational – objects that are lifted up Elastic – e.g. from springs Thermal – from hot objects Magnetic – objects in magnetic fields Electrostatic – charged objects Nuclear – stored within a nucleus
|
In any process energy is never created or destroyed. (It is just transferred from one store to another.)
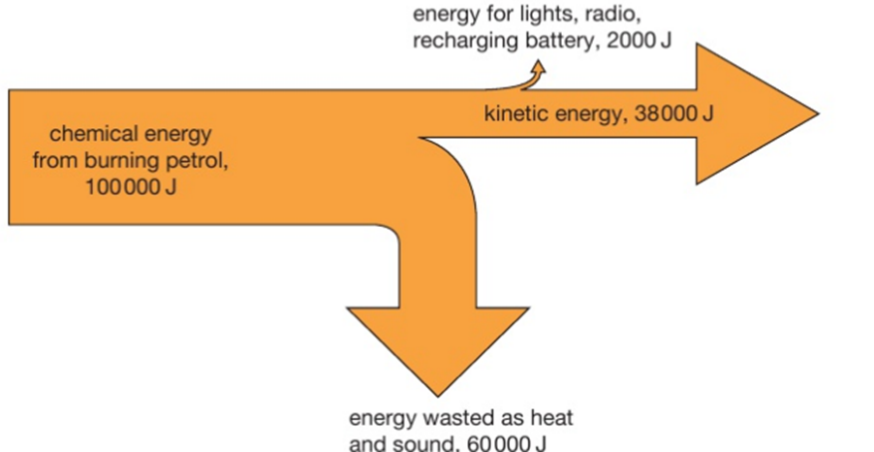
The energy flow is shown by arrows whose width is proportional to the amount of energy involved. The wasted and useful energy outputs are shown by different arrows.
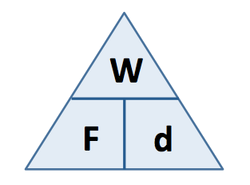
Work Done (J) = Force (N) x distance moved (m)
|
Work done = energy transferred |
Gravitational potential energy (J) = Mass (kg) x gravitational field strength (N/kg) x height (m)
Kinetic energy (J) = 0.5 x mass (kg) x velocity (m/s) 2
Because energy is conserved the decrease in GPE = increase in KE, for a falling object if no energy is lost to the surroundings
power is the rate of transfer of energy, or the rate of work done. so p = E/t
The units for:
temperature: degree Celsius (°C) or Kelvin (K)
Energy: Joule (J)
mass: Kilogram (kg)
density: kilogram/metre cubed (kg/m3)
distance: metre (m)
area: metre squared (m2)
volume: metre cubed (m3)
velocity: metre per second (m/s)
acceleration: metre per second squared (m/s2)
force: newton (N)
pressure: pascal (Pa)
the unit for
specific heat capacity: joules/kilogram degree Celsius (J/kg °C)
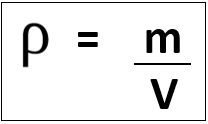
Units of density depend on units used for mass and volume:
E.g. mass in [g] and volume in [cm3] gives density in [g/cm3], however mass in [kg] and volume in [m3] gives density in [kg/m3].
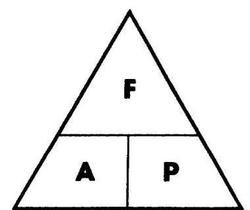
pressure (Pa) = force (N)/ area (m2)
Pressure difference [Pa] = Density [kg/m3] x g [N/kg] x Height [m]
ΔP = ρ g h
P1 – Patm = ρ g h
P1 = ρ g h + Patm
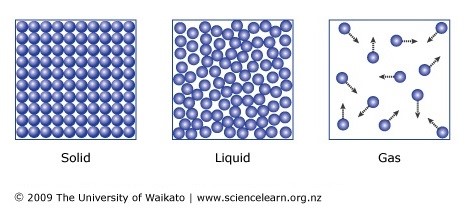
solids:
liquids:
gasses:
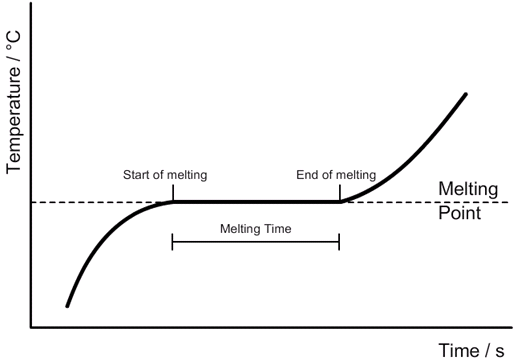
Specific heat capacity: