Acids & Salts (Triple) quiz
Finding the formula of a metal oxide experimentally
The formulae of metal oxides can be found experimentally by reacting a metal with oxygen and recording the mass changes.
Example: When magnesium is burned in air, it reacts with oxygen (O2) to form magnesium oxide (MgO).
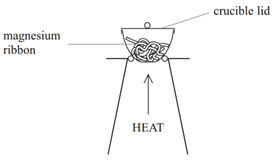
Method:
• Weigh a crucible and lid
• Place the magnesium ribbon in the crucible, replace the lid, and reweigh
• Calculate the mass of magnesium
(mass of crucible + lid + Magnesium – mass of crucible + lid)
• Heat the crucible with lid on until the magnesium burns
(lid prevents magnesium oxide escaping therefore ensuring accurate results)
• Lift the lid from time to time (this allows air to enter)
• Stop heating when there is no sign of further reaction
(this ensures all Mg has reacted)
• Allow to cool and reweigh
• Repeat the heating , cooling and reweigh until two consecutive masses are the same
(this ensures all Mg has reacted and therefore the results will be accurate)
• Calculate the mass of magnesium oxide formed (mass of crucible + lid + Magnesium oxide – mass of crucible + lid)
Finding the formula of a salt containing water of crystallisation
When some substances crystallise from solution, water becomes chemically bound up with the salt. This is called water of crystallisation and the salt is said to be hydrated. For example, hydrated copper sulfate has the formula CuSO4.5H2O which formula indicates that for every CuSO4 in a crystal there are five water (H2O) molecules.
When you heat a salt that contains water of crystallisation, the water is driven off leaving the anhydrous (without water) salt behind. If the hydrated copper sulfate (CuSO4.5H2O) are strongly heated in a crucible then they will break down and the water lost, leaving behind anhydrous copper sulfate (CuSO4). The method followed is similar to that for metal oxides, as shown above. The difference of mass before and after heating is the mass of the water lost. These mass numbers can be used to obtain the formula of the salt.
| Name of Ion | Formula | Charge |
|---|---|---|
| Sulfate | SO42- | -2 |
| Carbonate | CO32- | -2 |
| Nitrate | NO3- | -1 |
| Hydroxide | OH- | -1 |
| Ammonium | NH4+ | +1 |
| Hydrogen ion | H+ | +1 |
Ion charges on the periodic table
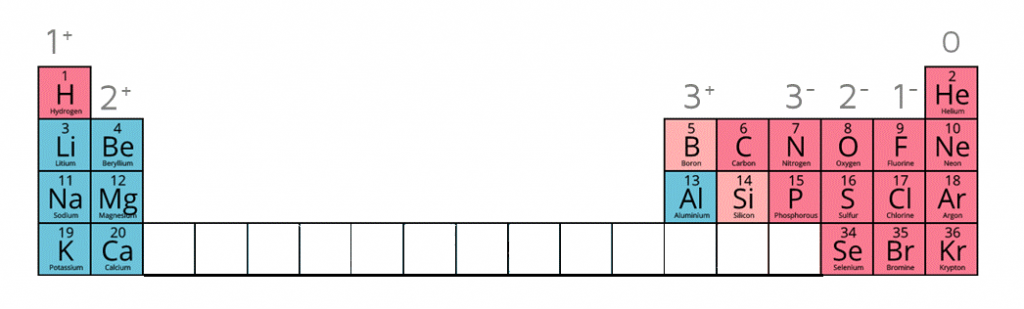
| Name of Ion | Formula | Charge |
|---|---|---|
| Sulfate | SO42- | -2 |
| Carbonate | CO32- | -2 |
| Nitrate | NO3- | -1 |
| Hydroxide | OH- | -1 |
| Ammonium | NH4+ | +1 |
| Silver ion | Ag+ | +1 |
| Zinc ion | Zn2+ | +2 |
| Hydrogen ion | H+ | +1 |
| Copper (II) ion | Cu2+ | +2 |
| Iron (II) ion | Fe2+ | +2 |
| Iron (III) ion | Fe3+ | +3 |
| Lead (II) ion | Pb2+ | +2 |
Ion charges on the periodic table

This excellent video from the lovely Tyler de Witt explains how to write the formula of an ionic compound:
This video shows how to work out the formula of a compound which contains polyatomic ions:
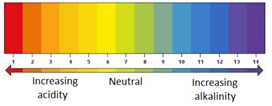
The pH scale ranges from 0 to 14, and tells you how acidic or how alkaline a solution is.
| strongly acidic | weakly acidic | neutral | weakly alkaline | strongly alkaline | |
|---|---|---|---|---|---|
| pH | 0-3 | 4-6 | 7 | 8-10 | 11-14 |
An indicator is a substance that has more than one colour form depending on the pH.
Universal indicator is a mixture of different dyes which change colour in a gradual way over a range of pH.
An acid is source of hydrogen ions (H+).
An alkali is source of hydroxide ions (OH–).
Metal oxides, metal hydroxides and ammonia (NH₃) are called bases.
Bases neutralise acids by combining with the hydrogen ions in them.
The key reaction is:
acid + base → salt + water
An example of this is:
sulfuric acid + copper oxide → copper sulfate + water
H₂SO₄ + CuO → CuSO₄ + H₂O
| Salt | Solubility | Exceptions |
|---|---|---|
| sodium (Na+), potassium (K+) and ammonium (NH4+) | soluble | none |
| nitrates (NO3-) | soluble | none |
| chlorides (Cl-) | soluble | silver chloride (AgCl) and lead (II) chloride (PbCl2) |
| sulfates (SO42-) | soluble | barium sulfate (BaSO4), calcium sulfate (CaSO4) and lead (II) sulfate (PbSO4) |
| carbonates (CO32-) | insoluble | sodium carbonate (Na2CO3), potassium carbonate (K2CO3) and ammonium carbonate ((NH4)2CO3) |
| hydroxides (OH-) | insoluble | sodium hydroxide (NaOH), potassium hydroxide (KOH) and calcium hydroxide (Ca(OH)2) (calcium hydroxide is slightly soluble) |
An acid is a proton (H⁺) donor.
A base is a proton (H⁺) acceptor.
A proton is the same as a hydrogen ion. A good way to think about that is to realise that a hydrogen atom is just one proton and zero neutrons surrounded by only one electron. If that atom becomes an ion by the removal of the electron, then only one proton is left.
When sulfuric acid reacts with copper (II) oxide (CuO):
Cu²⁺O²⁻ (s) + H₂SO₄ (aq) → Cu²⁺ (aq) + SO₄²⁻ (aq) + H₂O (l)
H₂SO₄ is an acid. It donates protons (H⁺) to CuO, the base.
An acid is a proton donor.
A base is a proton acceptor.
A proton is the same as a hydrogen ion. A good way to think about that is to realise that a hydrogen atom is just one proton and zero neutrons surrounded by only one electron. If that atom becomes an ion by the removal of the electron, then only one proton is left.
Acid reactions summary
alkali + acid → water + salt
base + acid → water + salt
carbonate + acid → water + salt + carbon dioxide
metal + acid → salt + hydrogen
To assist remembering this list, many pupils find it useful to remember this horrid looking but very effective mnemonic:
AAWS
BAWS
CAWS CoD
MASH
Acids are a source of hydrogen ions (H⁺) when in solution. When the hydrogen in an acid is replaced by a metal, the compound is called a salt. The name of the salt depends on the acid used. For example if sulfuric acid is used then a sulfate salt will be formed.
| Parent acid | Formula | Salt | Formula ion |
|---|---|---|---|
| sulfuric acid | H2SO4 | sulfate | SO42- |
| hydrochloric acid | HCl | chloride | Cl- |
| nitric acid | HNO3 | nitrate | NO3- |
Acid + Alkali and Acid + Base
A base is a substance that can neutralise an acid, forming a salt and water only.
Alkalis are soluble bases. When they react with acids, a salt and water is formed. The salt formed is often as a colourless solution. Alkalis are a source of hydroxide ions (OH⁻) when in solution.
alkali + acid → water + salt
base + acid → water + salt
Examples of acid + alkali reactions:
Example of an acid + base reaction:
CuO (s) + H₂SO₄ (aq) → CuSO₄ (aq) + H₂O (l)
Acid + Carbonate
carbonate + acid → water + salt + carbon dioxide
A carbonate is a compound made up of metal ions and carbonate ions. Examples of metal carbonates are sodium carbonate, copper carbonate and magnesium carbonate.
When carbonates react with acids, bubbling is observed which is the carbon dioxide being produced. If the acid is in excess the carbonate will disappear.
Examples of acid + carbonate reactions:
Acid + Metal
metal + acid → salt + hydrogen
Metals will react with an acid if the metal is above hydrogen in the reactivity series.
When metals react with acids, bubbling is observed which is the hydrogen being produced. If the acid is in excess the metal will disappear.
Examples of acid + metal reactions:
A base is a substance that neutralises an acid by combining with the hydrogen ions in them to produce water.
A base usually means a metal oxide, a metal hydroxide or ammonia.
Alkalis are bases which are soluble in water.
Some metal oxides are soluble in water and react with it to form solutions of metal hydroxides. For example:
Na₂O (s) + H₂O (l) → 2NaOH (aq)
Apart from this and other group 1 oxides (such as potassium oxide) most other metal oxides are not soluble in water.
One exception is calcium oxide which does dissolve slightly in water to form calcium hydroxide (known as limewater):
CaO (s) + H₂O (l) → Ca(OH)₂ (aq)
Ammonia is another base. Ammonia reacts with water to form ammonium ions and hydroxide ions:
NH₃ (aq) + H₂O (l) ⇋ NH₄⁺ (aq) + OH⁻ (aq)
All the solutions produced here contain hydroxide ions (OH⁻) so they are all alkalis.
Excess Solid Method:
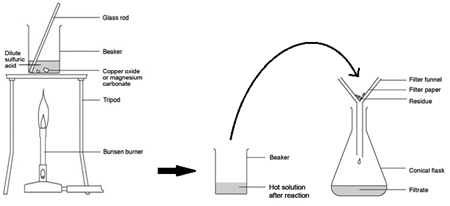
Preparing pure dry crystals of copper sulfate (CuSO4) from copper oxide (CuO) and sulfuric acid (H2SO4)
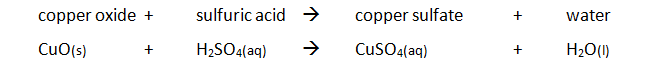
| Step | Explanation |
|---|---|
| Heat acid (H2SO4) in a beaker | Speeds up the rate of reaction |
| Add base (CuO) until in excess (no more copper oxide dissolves) and stir with glass rod | Neutralises all the acid |
| Filter the mixture using filter paper and funnel | Removes any excess copper oxide |
| Gently heat the filtered solution (CuSO4) | To evaporate some of the water |
| until crystals form on a glass rod | Shows a hot saturated solution formed |
| Allow the solution to cool so that hydrated crystals form | Copper sulfate less soluble in cold solution |
| Remove the crystals by filtration | Removes crystals |
| Dry by leaving in a warm place | Evaporates the water |
Titration Method:
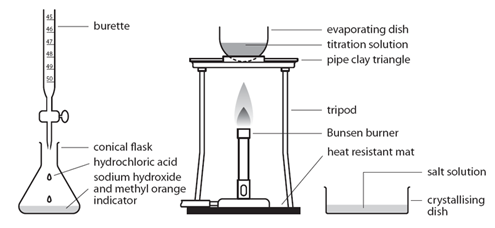
Preparing pure dry crystals of sodium chloride (NaCl) from hydrochloric acid (HCl) and sodium hydroxide (NaOH)
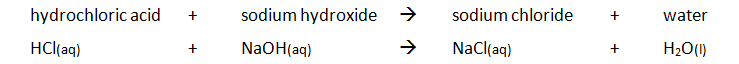
Before the salt preparation is carried out using the below method, the volume of acid that exactly reacts with 25cm3 of the alkali is found by titration using methyl orange indicator.
| Step | Explanation |
|---|---|
| Pipette 25cm3 of alkali (NaOH) into a conical flask | Accurately measures the alkali (NaOH) |
| Do not add indicator | Prevents contamination of the pure crystals with indicator |
| Using the titration values, titrate the known volume acid (HCl) into conical flask containing alkali | Exactly neutralises all of the alkali (NaOH) |
| Transfer to an evaporating basin & heat the solution | Forms a hot saturated solution (NaCl(aq)) |
| Allow the solution to cool so that hydrated crystals form | Sodium chloride is less soluble in cold water |
| Remove the crystals by filtration and wash with distilled water | Removes any impurities |
| Dry by leaving in a warm place | Evaporates the water |
(Note – This process could be reversed with the acid in the pipette and the alkali in the burette)
How to select the right method for preparing a salt:
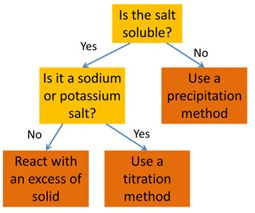
Precipitation Method:
Preparing pure dry crystals of silver chloride (AgCl) from silver nitrate solution (AgNO3) and potassium chloride solution (KCl)
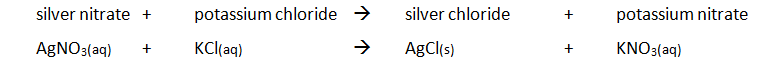
| Step | Explanation |
|---|---|
| Mix the two salt solutions together in a beaker | Forms a precipitate of an insoluble salt (AgCl) |
| Stir with glass rod | Make sure all reactants have reacted |
| Filter using filter paper and funnel | Collect the precipitate (AgCl) |
| Wash with distilled water | Removes any the other soluble salts (KNO3) |
| Dry by leaving in a warm place | Evaporates the water |
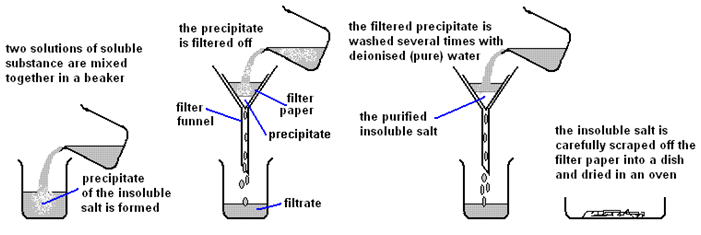
Excess Solid Method:
Preparing pure dry crystals of copper sulfate (CuSO4) from copper oxide (CuO) and sulfuric acid (H2SO4)
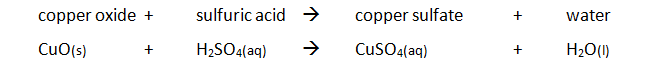
| Step | Explanation |
|---|---|
| Heat acid (H2SO4) in a beaker | Speeds up the rate of reaction |
| Add base (CuO) until in excess (no more copper oxide dissolves) and stir with glass rod | Neutralises all the acid |
| Filter the mixture using filter paper and funnel | Removes any excess copper oxide |
| Gently heat the filtered solution (CuSO4) | To evaporate some of the water |
| until crystals form on a glass rod | Shows a hot saturated solution formed |
| Allow the solution to cool so that hydrated crystals form | Copper sulfate less soluble in cold solution |
| Remove the crystals by filtration | Removes crystals |
| Dry by leaving in a warm place | Evaporates the water |

Objective: prepare a pure, dry sample of lead (II) sulfate (PbSO₄).
Preparing a pure, dry sample of lead (II) sulfate (PbSO₄) from lead (II) nitrate solution (Pb(NO₃)₂) and sodium sulfate solution (Na₂SO₄).
Pb(NO₃)₂ (aq) + Na₂SO₄ (aq) → PbSO₄ (s) + 2NaNO₃ (aq)
Tests for gases
| Gas | Test | Result if gas present |
|---|---|---|
| hydrogen (H2) | Use a lit splint | Gas pops |
| oxygen (O2) | Use a glowing splint | Glowing splint relights |
| carbon dioxide (CO2) | Bubble the gas through limewater | Limewater turns cloudy |
| ammonia (NH3) | Use red litmus paper | Turns damp red litmus paper blue |
| chlorine (Cl2) | Use damp litmus paper | Turns damp litmus paper white (bleaches) |
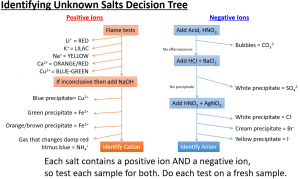
A flame test is used to show the presence of certain metal ions (cations) in a compound.
Properties of the platinum or nichrome wire is:
When put into a roaring bunsen burner flame on a nichrome wire, compounds containing certain cations will give specific colours as follows.
| Ion | Colour in flame test |
|---|---|
| lithium (Li⁺) | red |
| sodium (Na⁺) | yellow |
| potassium (K⁺) | lilac |
| calcium (Ca²⁺) | orange-red |
| copper (II) (Cu²⁺) | blue-green |

Describe tests for the cations Cu2+, Fe2+ and Fe3+, using sodium hydroxide solution
First, add sodium hydroxide (NaOH), then observe the colour:

Underneath is Sodium Hydroxide added to:
Iron (II) Ions
Iron (III) Ions
Copper (II) Ions
Ammonia
Describe tests for anions: Carbonate ions (CO32-)
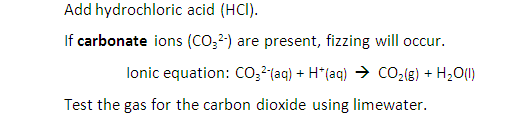
Describe tests for anions: Halide ions (Cl–, Br– and I–)
Underneath are the tests for:
chloride ion test, bromide ion test, iodide ion test, sulfate ion test, carbonate ion test

Describe tests for anions: Sulfate ions (SO42–)

Describe tests for anions: Carbonate ions (CO32-)
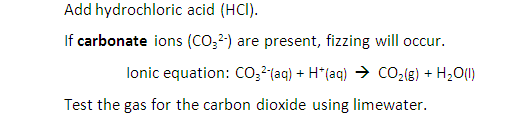
Underneath are the tests for:
Carbonate ions
sulfate ions
chloride ions
bromide ions
iodide ions
Here is summary of “testing for ions” (otherwise known as qualitative analysis). These “tests for ions” are commonly asked in iGCSE Edexcel Chemistry.
Each ionic compound contains positive ions and negative ions. To use the decision tree, start at the top and if a test is negative then move down to the next test.