Alcohols & Carboxylic Acids quiz
A good way to remember the names of organic molecules is to make up a silly mnemonic where the first letter of each word matches the first letter of the organic molecules. For example the first 10 alkanes in order are , Methane, Ethane, Propane, Butane, Pentane, Hexane, Heptane, Octane, Nonane and Decane. These can be memorised with “Many elephants prefer blue pinapples. However hungry orangutans never do.” This isn’t a very good mnemonic, but it is the one I made up for myself when I was at school, and you are best making up your own. The sillier, smellier and more colourful the better.
The names of organic molecules are based on the number of carbon atoms in the longest chain. This chain is the longest consecutive line of carbon atoms, even if this line bends.
| The name is based on the number of carbon atoms in the longest chain |
|---|
| 1 Meth- |
| 2 Eth- |
| 3 Prop- |
| 4 But- |
| 5 Pent- |
| 6 Hex- |
| 7 Hept- |
| 8 Oct- |
| 9 Non- |
| 10 Dec- |
Hydrocarbons are molecules which contain only hydrogen and carbon.
Naming straight-chain alkanes
The simplest hydrocarbons are alkanes. They contain only single bonds, and have “-ane” in the name.
For example, the displayed formula of ethane (C₂H₆) is:
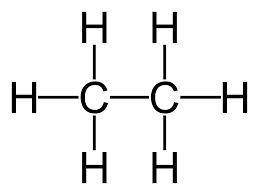
The name “ethane” contains “eth-” because there are 2 carbon atoms in the longest chain, and the name contains “-ane” because the molecule only has single bonds so is an alkane.
Another example is pentane (C₅H₁₂) which has the displayed formula:
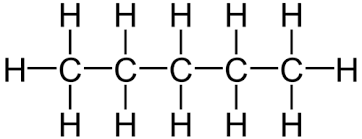
The name “pentane” contains “pent-” because there are 5 carbon atoms in the longest chain, and the name contains “-ane” because the molecule only has single bonds, so is an alkane.
Remember, it does not matter if the longest consecutive line of carbons bends around. For example the displayed formula below still shows a very normal molecule of pentane (5 carbons in a row). Pentane is not normally drawn with the longest chain of carbons bent around because it could be confusing.
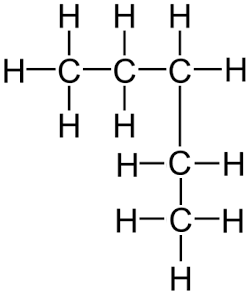
You might also see the bonds drawn at angles. Don’t worry, the displayed formula below is still pentane, as can be seen by the fact there are 5 carbon atoms in the longest chain, surrounded by hydrogen atoms bonded to the carbon atoms by single bonds.
A shorter way to express the detailed structure of an organic molecule is the structural formula. The structural formula for pentane is CH₃-CH₂-CH₂-CH₂-CH₃, which tells us the same information about the molecule as does the displayed formula, without the hassle of having to draw all the bonds or all the hydrogen atoms.
Naming straight-chain alkenes
Another simple group of hydrocarbons is the alkenes. They contain a carbon-to-carbon double bond, which also means they have two fewer hydrogen atoms than their corresponding alkane. An alkene has “-ene” in its name.
For example, the displayed formula for ethene (C₂H₄) is:
and the displayed formula of propene (C₃H₆) is:
With longer alkene molecules the double bond might appear in different locations of the carbon chain, so the name needs to be a little bit more complicated to be able to describe these differences clearly. A number is added in the middle of the name to indicate at which carbon the double bond starts.
So the displayed formula of pent-1-ene is:
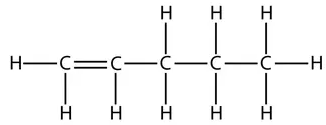
and this is the displayed formula of pent-2-ene:
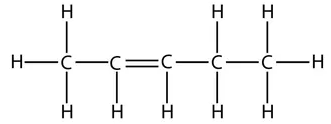
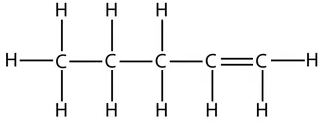
However, take care that when counting which carbon has the double bond. The numbers start from the end that produces the smallest numbers in the name. For example, this is the displayed formula for pent-1-ene again, but just drawn the other way round. It is still pent-1-ene (you can’t get pent-4-ene):
Naming straight-chain alcohols
We get the same pattern all over again with the group of organic molecules called alcohols, which are recognised by an -OH functional group. For example here is the displayed formula for ethanol, which has 2 carbon atoms in the longest chain:
and here is the displayed formula for butanol:
Summary of naming simple straight-chain organic molecules
The following table summarises the naming of some of the straight-chain alkanes, alkenes and alcohols, giving a name and a molecular formula for each:
| Carbons in longest chain | Alkanes | Alkenes | Alcohols |
|---|---|---|---|
| 1 | methane, CH₄ | - | methanol, CH₄O |
| 2 | ethane, C₂H₆ | ethene, C₂H₄ | ethanol, C₂H₆O |
| 3 | propane, C₃H₈ | propene, C₃H₆ | propanol, C₃H₈O |
| 4 | butane, C₄H₁₀ | butene, C₄H₈ | butanol, C₄H₁₀O |
Naming branched alkanes and alkenes
The naming conventions for organic molecules cover more than the straight chain molecules. Branched molecules are named depending on the number of carbon atoms in the branch. A branch with 1 carbon is called “methyl” and a branch with 2 carbons is called “ethyl”. This is similar to the conventions covered above, plus the “-yl-” bit just says it is a branch.
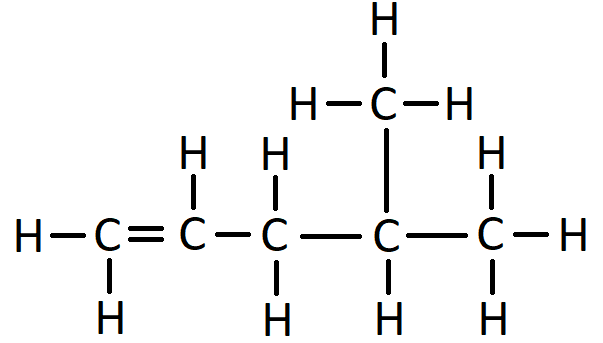
For example, this is the displayed formula for 2-methyl hexane:
In the name 2-methyl hexane, the number 2 indicates that when counting along the longest carbon chain the methyl branch comes off the second carbon atom. The “methyl” bit of the name says there is one branch of 1 carbon. The “hex” bit of the name says the longest consecutive chain of carbon atoms is 6. The “ane” bit says the molecule has only single bonds.
When counting along the carbon atoms of the longest chain to work out the name, the numbering of carbon atoms starts from the end nearest to the branch. Another way to put this is that the name is given such that the numbers in the name are as low as possible. For example, here is the displayed formula for 4-ethyl octane:
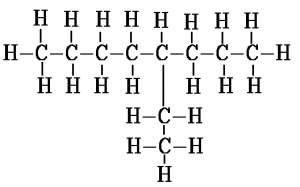
Another example, this time with 2 methyl branches coming off the second and third carbons of the chain, is 2,3-dimethyl hexane. The “di” in the name indicates there are two methyl groups. This is the same way in which “di” indicates there are two oxygen atoms in carbon dioxide.
Another example of how the naming convention works for branches is 2,2-dimethyl hexane:
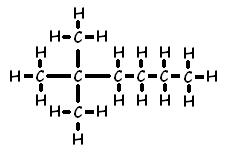
This naming of branches also applies to alkenes. Here is the displayed formula of 4-methylpent-1-ene:
The member of the homologous series called Alcohols have names which end in “ol”. Examples are methanol, ethanol and propanol.
Alcohols all contain an -OH functional group attached to a hydrocarbon chain.
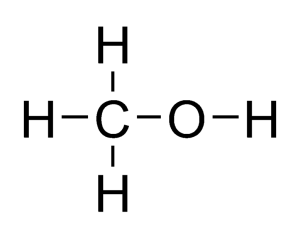
Structural formula and displayed formula for methanol:
CH₃-OH
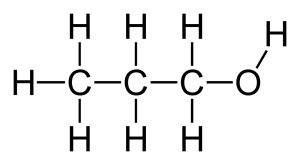
Structural formula and displayed formula for ethanol:
CH₃-CH₂-OH (or simply C₂H₅OH)
Structural formula and displayed formula for propan-1-ol:
CH₃-CH₂-CH₂-OH
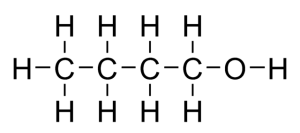
Structural formula and displayed formula for butan-1-ol:
CH₃-CH₂-CH₂-CH₂-OH
This video introduces alcohols:
1) Ethanol can be oxidised by complete combustion. With excess oxygen the complete combustion of ethanol (C₂H₅OH) in air produces carbon dioxide and water:
C₂H₅OH (l) + 3O₂ (g) → 2CO₂ (g) + 3H₂O (l)
2) Ethanol can be oxidised in air in the presence of microorganisms (‘microbial oxidation’) to form ethanoic acid (CH₃COOH).
3) Ethanol can be oxidised by heating with the oxidising agent potassium dichromate(VI) (K₂Cr₂O₇) in dilute sulfuric acid (H₂SO₄).
In the equation below, [O] means oxygen from an oxidising agent.
CH₃CH₂OH + 2[O] → CH₃COOH + H₂O
This mixture starts orange but when the reaction happens turns green which indicates the presence of Cr³⁺ ions which are formed when the potassium dichromate(VI) is reduced.
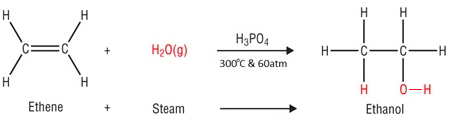 In the hydration of ethene, ethanol is made by passing ethene and steam over a catalyst.
In the hydration of ethene, ethanol is made by passing ethene and steam over a catalyst.
Water is added to ethene, this is known as hydration.
Conditions
Catalyst: Phosphoric acid (H3PO4)
Temperature: 300°C
Pressure: 60 atm
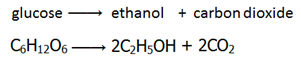 Fermentation is the conversion of sugar, e.g. glucose into ethanol by enzymes from yeast.
Fermentation is the conversion of sugar, e.g. glucose into ethanol by enzymes from yeast.
Conditions
Catalyst: Zymase (enzyme found in yeast)
Temperature: 30°C – The process is carried out at low temperatures as not to denature the enzymes.
Other: Anaerobic (no oxygen present) – if oxygen were present, the yeast produce carbon dioxide and water instead of ethanol.
In the production of ethanol the process of fermentation is carried out at a low temperature (30⁰-40⁰).
Above 40⁰ the enzymes would permanently lose their structure (denature).
At a temperature lower than 30⁰ the process would be too slow.
Fermentation is conducted in the absence of air. In the presence of air (aerobic conditions), enzymes in the yeast produce carbon dioxide and water instead of ethanol.
Also, in the presence of air, the ethanol can oxidise to ethanoic acid.
Carboxylic acids contain the functional group -COOH
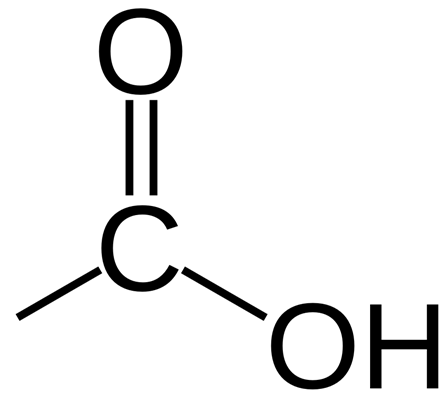
An example of a carboxylic acid is butanoic acid:
The four simplest carboxylic acids are methanoic acid, ethanoic acid, propanoic acid and butanoic acid.
Methanoic acid
Displayed formula:
Molecular formula: CH₂O₂
Structural formula: HCOOH
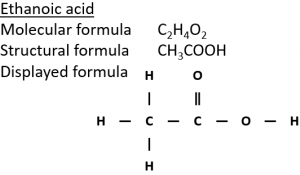
Ethanoic acid
Displayed formula: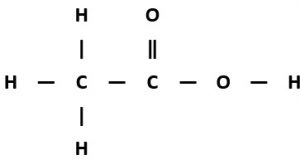
Molecular formula: C₂H₄O₂
Structural formula: CH₃COOH
Propanoic acid
Displayed formula:
Molecular formula: C₃H₆O₂
Structural formula: CH₃CH₂COOH
Butanoic acid
Displayed formula:
Molecular formula: C₄H₈O₂
Structural formula: CH₃CH₂CH₂COOH
This video introduces carboxylic acids:
Dilute carboxylic acids react with metals in the same way as other dilute acids (e.g. hydrochloric acid) only more slowly.
For example, dilute ethanoic acid reacts with magnesium with a lot of fizzing to produce a salt and hydrogen, leaving a colourless solution of magnesium ethanoate:
magnesium + ethanoic acid → magnesium ethanoate + hydrogen
Mg (s) + 2CH₃COOH (aq) → (CH₃COO)₂Mg (aq) + H₂ (g)
Dilute carboxylic acids react with metal carbonates as they do with other acids, to give a salt, carbon dioxide and water.
For example, dilute ethanoic acid reacts with sodium carbonate with a lot of fizzing to produce a salt, carbon dioxide and water, leaving a colourless solution of sodium ethanoate:
sodium carbonate + ethanoic acid → sodium ethanoate + carbon dioxide + water
Na₂CO₃ (s) + 2CH₃COOH (aq) → 2CH₃COONa (aq) + CO₂ (g) + H₂O (l)
As can be seen in the examples above the charge on the ethanoate ion is -1.
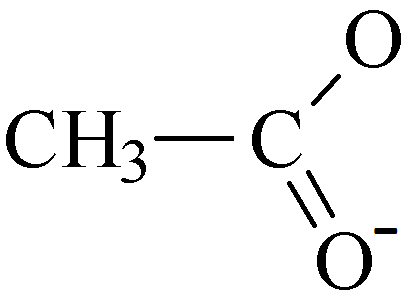
Vinegar is an aqueous solution containing ethanoic acid (CH₃COOH).
Esters contain the functional group -COO-
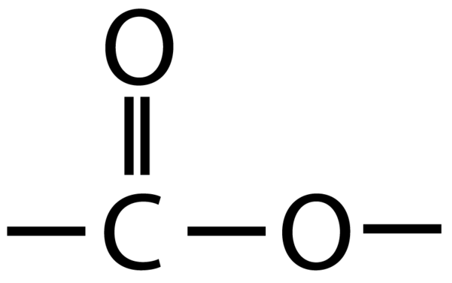
An example of an ester is ethyl ethanoate:
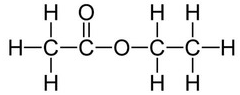
Ethyl ethanoate is the ester produced when ethanol and ethanoic acid react in the presence of an acid catalyst.
ethanoic acid + ethanol ⇋ ethyl ethanoate + water
CH₃COOH (l) + CH₃CH₂OH (l) ⇋ CH₃COOCH₂CH₃ (l) + H₂O (l)
The ethyl ethanoate produced is an ester.
The reaction is called esterification.
The reaction can also be described as a condensation reaction because water is made when two molecules join together.
Ethyl ethanoate is an ester.
Structural formula: CH₃COOCH₂CH₃
Displayed formula:
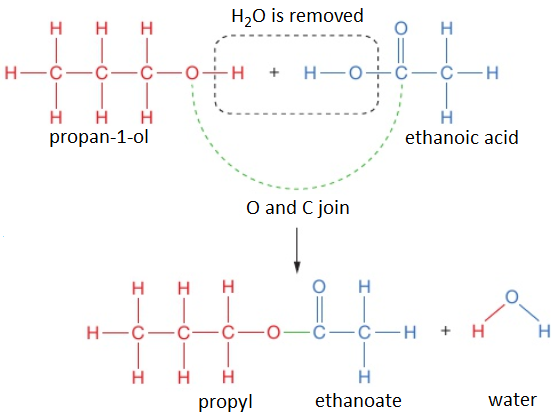
To work out the structure of the ester formed when an alcohol reacts with a carboxylic acid, it is easiest to first draw the structures of alcohol and acid and then remove the H₂O to see what is left when the molecules join.
For example, propan-1-ol and ethanoic acid react together to form propyl ethanoate and water.
propan-1-ol + ethanoic acid → propyl ethanoate + water
CH₃CH₂CH₂OH (l) + CH₃COOH (l) → CH₃COOCH₂CH₂CH₃ (l) + H₂O (l)
The displayed formula for esters is typically written to show the part which came from the carboxylic acid on the left:
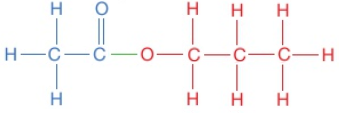
This is still called propyl ethanoate: the alcohol bit of the name (propyl) comes first and then the carboxylic acid bit (ethanoate), even though the displayed formula is typically written the other way around. Notice that the structural formulae for esters is also typically written with the carboxylic acid bit first, so propyl ethanoate is CH₃COOCH₂CH₂CH₃.
Esters are volatile compounds with distinctive smells. A volatile liquid is one that turns into to a vapour easily.
Esters are used as food flavourings and in perfumes.
Esters are often described as having a sweet, fruity smell. They typically smell of bananas, raspberries, pears or other fruit because esters occur in all these natural products.
Heating a mixture of ethanoic acid and ethanol produces a liquid called ethyl ethanoate. A few drops of concentrated sulfuric acid must be added for the reaction to work. The sulfuric acid acts as a catalyst.
ethanoic acid + ethanol ⇋ ethyl ethanoate + water
CH₃COOH (l) + CH₃CH₂OH (l) ⇋ CH₃COOCH₂CH₃ (l) + H₂O (l)
The ethyl ethanoate produced is an ester.
The reaction is called esterification.
The reaction can also be described as a condensation reaction because water is made when two molecules join together.
Notice that the reaction is reversible. Pure reactants are used to maximise the yield of ethyl ethanoate. Pure ethanoic acid is called glacial ethanoic acid.
[ Thanks to the amazing Ms B for this resource ]