Energetics (Triple) quiz
Exothermic: chemical reaction in which heat energy is given out.
Endothermic: chemical reaction in which heat energy is taken in.
(So, in an exothermic reaction the heat exits from the chemicals so temperature rises)
Calorimetry allows for the measurement of the amount of energy transferred in a chemical reaction to be calculated.
EXPERIMENT1: Displacement, dissolving and neutralisation reactions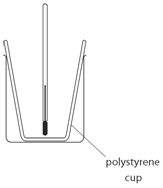
Example: magnesium displacing copper from copper(II) sulfate
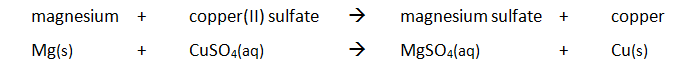
Method:
| Initial temp. of solution (oC) | Maximium temp. of solution (oC) | Temperature rise (oC) |
|---|---|---|
| 24.2 | 56.7 | 32.5 |
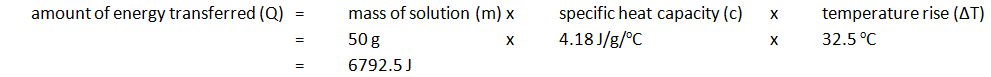
Note: mass of 50 cm3 of solution is 50 g
The cup used is polystyrene because:
polystyrene is an insulator which reduces heats loss
EXPERIMENT2: Combustion reactions
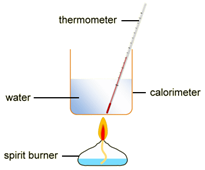
To measure the amount of energy produced when a fuel is burnt, the fuel is burnt and the flame is used to heat up some water in a copper container
Example: ethanol is burnt in a small spirit burner
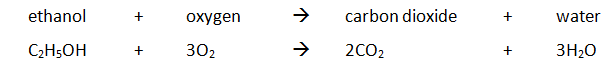
Method:
| Mass of water (g) | Initial temp of water (oC) | Maximum temp of water (oC) | Temperature rise (oC) | Initial mass of spirit burner + ethanol (g) | Final mass of spirit burner + ethanol (g) | Mass of ethanol burnt (g) |
|---|---|---|---|---|---|---|
| 100 | 24.2 | 54.2 | 30.0 | 34.46 | 33.68 | 0.78 |
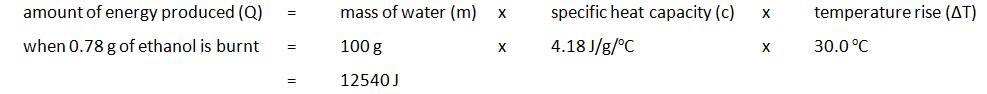
The amount of energy produced per gram of ethanol burnt can also be calculated:

Each type of chemical bond has a particular bond energy. The bond energy can vary slightly depending what compound the bond is in, therefore average bond energies are used to calculate the change in heat (enthalpy change, ΔH) of a reaction.
Example: dehydration of ethanol
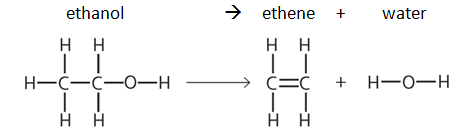
Note: bond energy tables will always be given in the exam, e.g:
| Bond | Average bond energy in kJ/mol |
|---|---|
| H-C | 412 |
| C-C | 348 |
| O-H | 463 |
| C-O | 360 |
| C=C | 612 |
So the enthalpy change in this example can be calculated as follows:
| Breaking bonds | Making bonds | ||
|---|---|---|---|
| Bonds | Energy (kJ/mol) | Bonds | Energy (kJ/mol) |
| H-C x 5 | (412 x 5) = 2060 | C-H x 4 | (412 x 4) = 1648 |
| C-C | 348 | C=C | 612 |
| C-O | 360 | O-H x 2 | (463 x 2) = 926 |
| O-H | 463 | ||
| Energy needed to break all the bonds | 3231 | Energy released to make all the new bonds | 3186 |
Enthalpy change, ΔH = Energy needed to break all the bonds - Energy released to make all the new bonds ΔH = 3231 – 3186 = +45 kJ/mol (ΔH is positive so the reaction is endothermic) |
|||
Here are a couple of videos explaining how to do bond energy calculations:
Calorimetry allows for the measurement of the amount of energy transferred in a chemical reaction to be calculated.
EXPERIMENT1: Displacement, dissolving and neutralisation reactions
Example: magnesium displacing copper from copper(II) sulfate
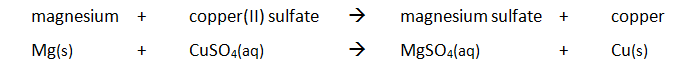
Method:
| Initial temp. of solution (oC) | Maximium temp. of solution (oC) | Temperature rise (oC) |
|---|---|---|
| 24.2 | 56.7 | 32.5 |
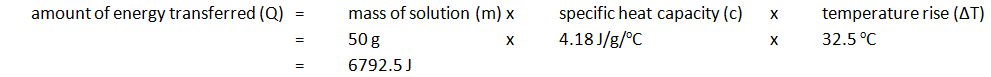
Note: mass of 50 cm3 of solution is 50 g
EXPERIMENT2: Combustion reactions

To measure the amount of energy produced when a fuel is burnt, the fuel is burnt and the flame is used to heat up some water in a copper container
Example: ethanol is burnt in a small spirit burner
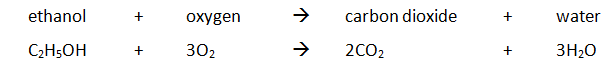
Method:
| Mass of water (g) | Initial temp of water (oC) | Maximum temp of water (oC) | Temperature rise (oC) | Initial mass of spirit burner + ethanol (g) | Final mass of spirit burner + ethanol (g) | Mass of ethanol burnt (g) |
|---|---|---|---|---|---|---|
| 100 | 24.2 | 54.2 | 30.0 | 34.46 | 33.68 | 0.78 |
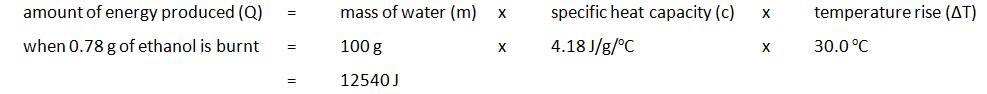
The amount of energy produced per gram of ethanol burnt can also be calculated:
