Equilibria (triple) quiz
Diffusion is the spreading out of particles in a gas or liquid. There is a net movement of particles from areas of high concentration to areas of low concentration until a uniform concentration is achieved.
i) dilution of coloured solutions
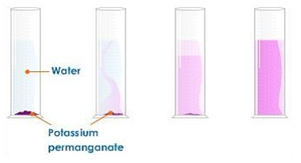 Dissolving potassium manganate(VII) in water demonstrates that the diffusion in liquids is very slow because there are only small gaps between the liquid particles into which other particles diffuse.
Dissolving potassium manganate(VII) in water demonstrates that the diffusion in liquids is very slow because there are only small gaps between the liquid particles into which other particles diffuse.
The random motion of particles cause the purple colour to eventually be evenly spread out throughout the water.
Adding more water to the solution causes the potassium manganate(VII) particles to spread out further apart therefore the solutions becomes less purple. This is called dilution.
ii) diffusion experiments
When ammonia gas and hydrogen chloride gas mix, they react together to form a white solid called ammonium chloride.
ammonia + hydrogen chloride –> ammonium chloride
NH3(g) + HCl(g) –> NH4Cl(s)
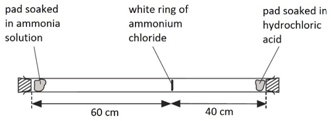
A cotton wool pad was soaked in ammonia solution and another was soaked in hydrogen chloride solution. The two pads were then put into opposite ends of a dry glass tube at the same time.
The white ring of ammonium chloride forms closer to the hydrochloric acid end because ammonia particles are lighter than hydrogen chloride particles and therefore travel faster.
Even though these particles travel at several hundred metres per second, it takes about 5 min for the ring to form. This is because the particles move in random directions and will collide with air particles in the tube.
This video is a great way to see the diffusion of particles in action:
Yield is how much product you get from a chemical reaction.
The theoretical yield is the amount of product that you would expect to get. This is calculated using reacting mass calculations.
In most chemical reactions, however, you rarely achieved your theoretical yield.
For example, in the following reaction:
CaCO3 –> CaO + CO2
You might expect to achieve a theoretical yield of 56 g of CaO from 100 g of CaCO3.
However, what if the actual yield is only 48 g of CaO.
By using the following formula, the % yield can be calculated:
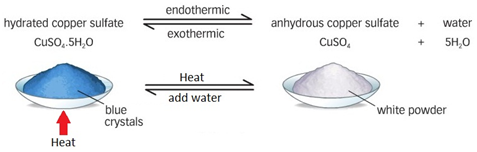
Add anhydrous copper (II) sulfate (CuSO4) to a sample.
If water is present the anhydrous copper (II) sulfate will change from white to blue.
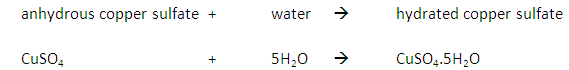
Exothermic: chemical reaction in which heat energy is given out.
Endothermic: chemical reaction in which heat energy is taken in.
(So, in an exothermic reaction the heat exits from the chemicals so temperature rises)
Calorimetry allows for the measurement of the amount of energy transferred in a chemical reaction to be calculated.
EXPERIMENT1: Displacement, dissolving and neutralisation reactions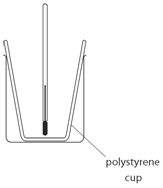
Example: magnesium displacing copper from copper(II) sulfate
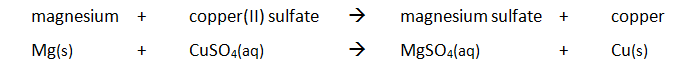
Method:
| Initial temp. of solution (oC) | Maximium temp. of solution (oC) | Temperature rise (oC) |
|---|---|---|
| 24.2 | 56.7 | 32.5 |
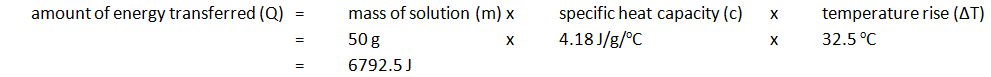
Note: mass of 50 cm3 of solution is 50 g
The cup used is polystyrene because:
polystyrene is an insulator which reduces heats loss
EXPERIMENT2: Combustion reactions
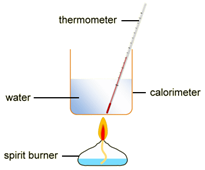
To measure the amount of energy produced when a fuel is burnt, the fuel is burnt and the flame is used to heat up some water in a copper container
Example: ethanol is burnt in a small spirit burner
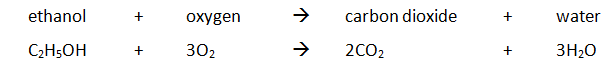
Method:
| Mass of water (g) | Initial temp of water (oC) | Maximum temp of water (oC) | Temperature rise (oC) | Initial mass of spirit burner + ethanol (g) | Final mass of spirit burner + ethanol (g) | Mass of ethanol burnt (g) |
|---|---|---|---|---|---|---|
| 100 | 24.2 | 54.2 | 30.0 | 34.46 | 33.68 | 0.78 |
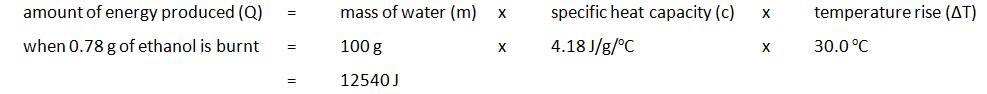
The amount of energy produced per gram of ethanol burnt can also be calculated:

The symbol ΔH is used to represent the change in heat (or enthalpy change) of a reaction.
ΔH is measured in kJ/mol (kilojoules per mole).
The change in heat (enthalpy change) can be represented on an energy level diagram. ΔH must also labelled.
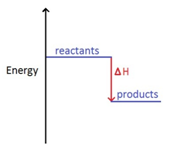
In an exothermic reaction, the reactants have more energy than the products.
Energy is given out in the form of heat which warms the surroundings.
ΔH is given a negative sign, because the reactants are losing energy as heat, e.g ΔH = -211 kJ/mol.
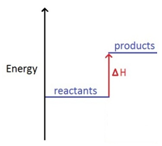
In an endothermic reaction, the reactants have less energy than the products.
Energy is taken in which cools the surroundings.
ΔH is given a positive sign, because the reactants are gaining energy, e.g ΔH = +211 kJ/mol.
Increasing the surface area of a solid:
Increasing the concentration of a solution or pressure of a gas:
Increasing the temperature:
A catalyst is a substance that increases the rate of a reaction, but is chemically unchanged at the end of the reaction.
Below is a diagram showing the reaction profile for the reaction of hydrogen with oxygen, which is EXOTHERMIC:
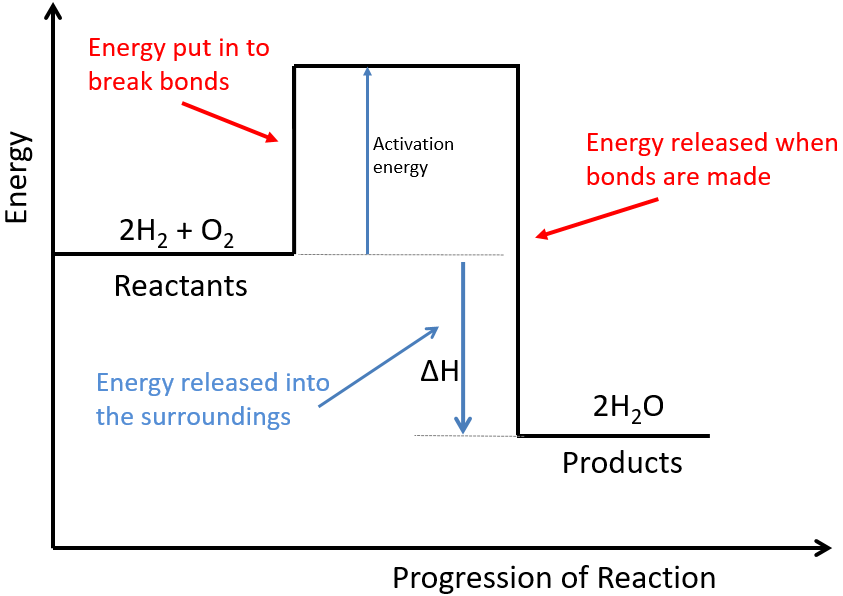
The activation energy is the minimum amount of energy required to start the reaction.
For an exothermic reaction, the products have less energy than the reactants. The difference between these energy levels is ΔH.
For an exothermic reaction, more energy is released when bonds are formed than taken in when bonds are broken.
Below is a diagram showing the reaction profile for the thermal decomposition of calcium carbonate, which is ENDOTHERMIC:
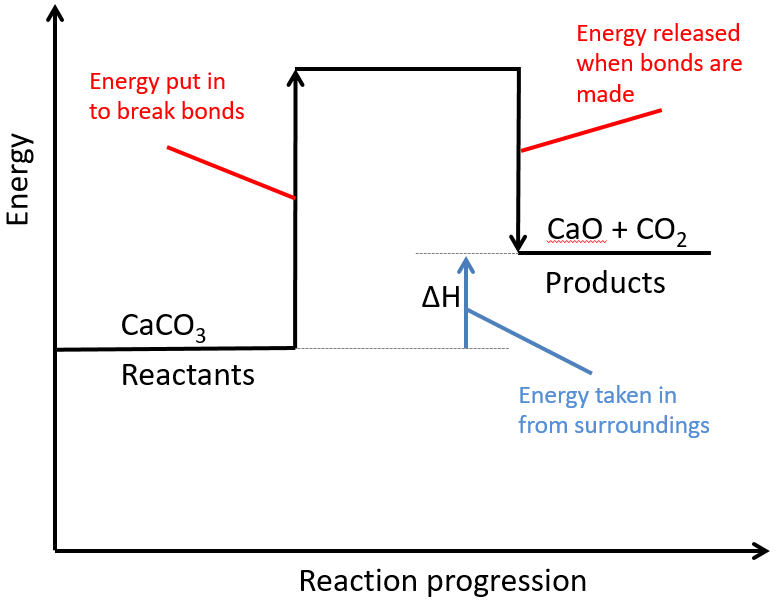
The activation energy is the minimum amount of energy required to start the reaction.
For an endothermic reaction, the products have more energy than the reactants. The difference between these energy levels is ΔH.
For an endothermic reaction, more energy is taken in to break bonds than is released when new bonds are formed.
Some reactions are reversible. This is indicated by the symbol ⇌
For example:
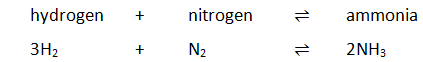
Dehydration of copper(II) sulfate
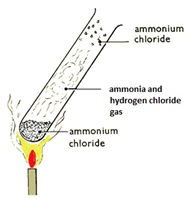 Heating ammonium chloride
Heating ammonium chloride
On heating, white solid ammonium chloride decomposes forming ammonia and hydrogen chloride gas. On cooling, ammonia and hydrogen chloride react to form a white solid of ammonium chloride:
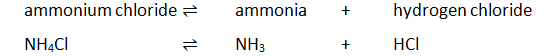
Here is a video introducing reversible reactions:
A dynamic equilibrium can only be achieved in a sealed container so substances cannot escape.
Features of a reaction mixture that is in dynamic equilibrium:
A catalyst is a substance which increases the rate of reaction without being chemically changed at the end of the reaction.
A reversible reaction is one where the forward reaction and the backward reaction happen simultaneously. For example:
3H₂ + N₂ ⇋ 2NH₃
In such a reaction a catalyst speeds up both the forward and the backward reactions. Hence, although the system will reach dynamic equilibrium more quickly, the addition of a catalyst will not affect the position of equilibrium.
In a reversible reaction the position of the equilibrium (the relative amounts of reactants and products) is dependent on the temperature and pressure of the reactants.
If the conditions of an equilibrium reaction are changed, the reaction moves to counteract that change.
Therefore by altering the temperature or pressure the position of the equilibrium will change to give more or less products.
Adding a catalyst does not affect the position of the equilibrium.
If a change in conditions moves equilibrium to the right, the yield of the substances on the right is increased.
Changing the temperature:
All reactions are exothermic in one direction and endothermic in the other way.
For this reaction the enthalpy change, ΔH is negative therefore the forward reaction is exothermic:
CO(g) + 2H2(g) ⇌ CH3OH(g) ΔH = –91 kJ/mol
If temperature is decreased the position of the equilibrium will shift to the right because it is an exothermic reaction.
For this reaction the enthalpy change, ΔH is positive therefore the forward reaction is endothermic:
CH4(g) + H2O(g) ⇌ CO(g) + 3H2(g) ΔH = +210 kJ mol–1
If temperature is increased the position of the equilibrium will shift to the right because it is an endothermic reaction.
Key point: an increase (or decrease) in temperature shifts the position of equilibrium in the direction of the endothermic (or exothermic) reaction
Changing the pressure:
Reactions may have more molecules of gas on one side than on the other.
For this reaction there are 2 molecules on the left and 4 molecules on the right:
CH4(g) + H2O(g) ⇌ CO(g) + 3H2(g) ΔH = +210 kJ mol–1
If the pressure is increased the position of the equilibrium will shift to the left because there are fewer molecules on the left-hand side.
For this reaction there are 3 molecules on the left and 1 molecule on the right
CO(g) + 2H2(g) ⇌ CH3OH(g) ΔH = –91 kJ/mol
If the pressure is decreased the position of the equilibrium will shift to the left because there are more molecules on the left-hand side.
Key point: an increase (or decrease) in pressure shifts the position of equilibrium in the direction that produces fewer (or more) moles of gas
This video explains how the position of equilibrium in a reversible reaction is affected by changes to temperature.
This video explains how the position of equilibrium in a reversible reaction is affected by changes to pressure.