Key Reactions quiz
There are several things in this video which are “beyond the exam spec” but there are loads of interesting bits…..
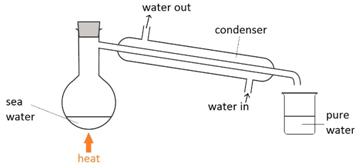
Simple distillation
This method is used to separate a liquid from a solution. For example: separating water from salt water.
The salt water is boiled. The water vapour condenses back into a liquid when passed through the condenser. The salt is left behind in the flask.
Note: cold water is passed into the bottom of the condenser and out through the top so that the condenser completely fills up with water.
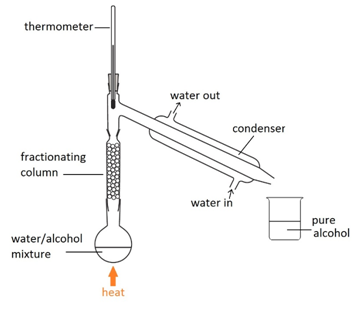
Fractional distillation
This method is used to separate a mixture of different liquids that have different boiling points. For example, separating alcohol from a mixture of alcohol and water.
Water boils at 100oC and alcohol boils at 78oC. By using the thermometer to carefully control of temperature of the column, keeping it at 78oC, only the alcohol remains as vapour all the way up to the top of the column and passes into the condenser.
The alcohol vapours then condense back into a liquid.
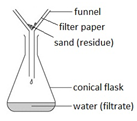 Filtration
Filtration
This method is used to separate an insoluble solid from a liquid. For example: separating sand from a mixture of sand and water.
The mixture is poured into the filter paper. The sand does not pass through and is left behind (residue) but the water passes through the filter paper and is collected in the conical flask (filtrate).
Crystallisation
This method is used to obtain a salt which contains water of crystallisation from a salt solution. For example: hydrated copper sulfate crystals (CuSO4.5H2O(s)) from copper sulfate solution (CuSO4(aq)).
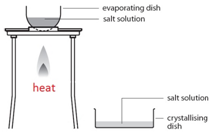 Gently heat the solution in an evaporating basin to evaporate some of the water
Gently heat the solution in an evaporating basin to evaporate some of the waterIf instead the solution is heated until all the water evaporates, you would produce a powder of anhydrous copper sulfate (CuSO4(s)).
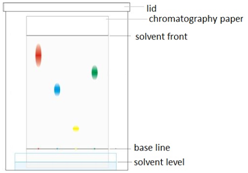
Paper chromatography
This method can be used to separate the parts of a mixture into their components. For example, the different dyes in ink can all be separated and identified.
The coloured mixture to be separated (e.g. a food dye) is dissolved in a solvent like water or ethanol and carefully spotted onto the chromatography paper on the baseline, which is drawn in pencil so it doesn’t ‘run or smudge’.
The paper is carefully dipped into the solvent and suspended so the baseline is above the liquid solvent, otherwise all the spots would dissolve in the solvent. The solvent is absorbed into the paper and rises up it as it soaks into the paper. The choice of solvent depends on the solubility of the dye. If the dye does not dissolve in water then normally an organic solvent (e.g. ethanol) is used.
As the solvent rises up the paper it will carry the dyes with it. Each different dye will move up the paper at different rates depending on how strongly they stick to the paper and how soluble they are in the solvent.
Below is the preparation of copper sulfate crystals (CuSO4.5H2O) through the process of filtration and crystallisation
Metals
Non – Metals
Example:
Sodium (Na) reacts with water (H2O) to produce a solution of sodium hydroxide (NaOH) and hydrogen gas (H2).
Word equation:
sodium + water –> sodium hydroxide + hydrogen
Writing the chemical equation
A chemical equation represents what happens in terms of atoms in a chemical reaction.
Step 1: To write a chemical equation we need to know the chemical formulae of the substances.
Na + H2O –> NaOH + H2
Step 2: The next step is to balance the equation: write a large number before each compound so the number of atoms of each element on the left hand side (reactants) matches the number on the right (products). This large number is the amount of each compound or element.
During this balancing stage the actual formulas for each compound must not be changed. Only the number of each compound changes.
2Na + 2H2O –> 2NaOH + H2
If asked for an equation, the chemical equation must be given.
State symbols are used to show what physical state the reactants and products are in.
| State symbols | Physical state |
|---|---|
| (s) | Solid |
| (l) | Liquid |
| (g) | Gas |
| (aq) | Aqueous solution (dissolved in water) |
Example:
A solid piece of sodium (Na) reacts with water (H2O) to produce a solution of sodium hydroxide (NaOH) and hydrogen gas (H2).
2Na(s) + 2H2O(l) –> 2NaOH(aq) + H2(g)
This excellent Tyler de Witt video is an introduction to balancing equations:
And here’s another of the lovely Tyler’s videos with some practice questions and answers on equation balancing:
When given this information of the following ions, it is possible to work out the formulae of ionic compounds which include these ions.
| Name of Ion | Formula | Charge |
|---|---|---|
| Sulfate | SO42- | -2 |
| Carbonate | CO32- | -2 |
| Nitrate | NO3- | -1 |
| Hydroxide | OH- | -1 |
| Ammonium | NH4+ | +1 |
| Silver ion | Ag+ | +1 |
| Zinc ion | Zn2+ | +2 |
| Hydrogen ion | H+ | +1 |
| Copper (II) ion | Cu2+ | +2 |
| Iron (II) ion | Fe2+ | +2 |
| Iron (III) ion | Fe3+ | +3 |
| Lead (II) ion | Pb2+ | +2 |
Ion charges on the periodic table
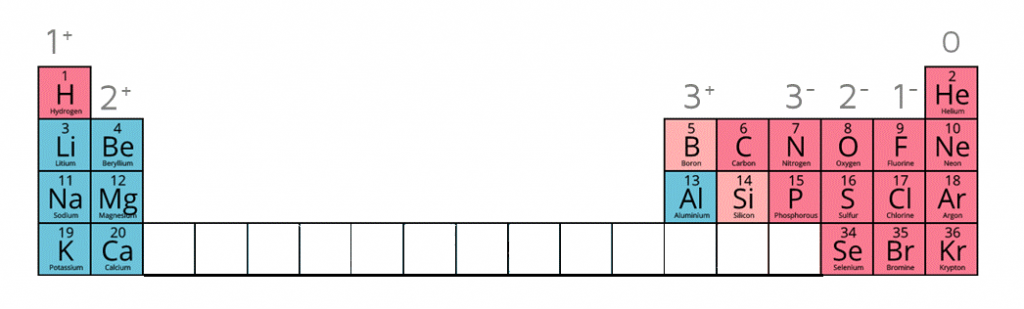
Writing the electron configuration of an atom allows you to work out the electron configuration of the ion and therefore the charge on the ion.
Examples:
Atom = Mg
Electron configuration = 2,8,2
remove the two electrons from the outer shell to achieve the same electron configuration as the nearest noble gas, Neon (Ne 2,8)
Ion = Mg2+
Atom = O
Electron configuration = 2,6
add two electrons to the outer shell to achieve the same electron configuration as the nearest noble gas, Neon (Ne 2,8)
Ion = O2- [2,8]2-
This video shows how to work out the formula of a compound which contains polyatomic ions:
Magnesium reacts with oxygen producing a bright white flame leaving behind a white ash of magnesium oxide.
magnesium + oxygen → magnesium oxide
2Mg (s) + O₂ (g) → 2MgO
MgO is a base, which can react with an acid to give a salt and water.
Hydrogen reacts with oxygen in an explosive reaction. This is the basis of the ‘squeak pop’ test for hydrogen in test tube. With larger quantities of hydrogen this explosion can be dangerous.
hydrogen + oxygen → water
2H₂ (g) + O₂ (g) → 2H₂O (l)
Sulfur reacts with oxygen producing a blue flame.
sulfur + oxygen → sulfur dioxide
S (s) + O₂ (g) → SO₂ (g)
When sulfur dioxide (SO₂) dissolves in water it forms an acidic solution of sulfurous acid:
SO₂ (g) + H₂O (l) → H₂SO₃ (aq)
Underneath are sodium, magnesium, iron, carbon, phosphorus, Sulphur burning in oxygen
This video shows the reaction with oxygen of various elements:
thermal decomposition is the process of breaking down by heating.
On heating metal carbonates thermal decompose into metal oxides and carbon dioxide.

Observation: green powder (CuCO3) changes to a black powder (CuO)
| Indicator | Colour in acidic solution | Colour in alkaline solution |
|---|---|---|
| Litmus | Red | Blue |
Indicators are substances which change colour in response to a change in pH (acid or alkali).
| Indicator | Colour in acidic solution [H+] | Colour in alkaline solution [OH-] |
|---|---|---|
| Litmus | Red | Blue |
| Methyl orange | Red | Yellow |
| Phenolphthalein | Colourless | Pink |
Methyl orange is orange in a neutral solution
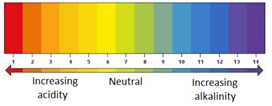
The pH scale ranges from 0 to 14, and tells you how acidic or how alkaline a solution is.
| strongly acidic | weakly acidic | neutral | weakly alkaline | strongly alkaline | |
|---|---|---|---|---|---|
| pH | 0-3 | 4-6 | 7 | 8-10 | 11-14 |
An indicator is a substance that has more than one colour form depending on the pH.
Universal indicator is a mixture of different dyes which change colour in a gradual way over a range of pH.
An acid is source of hydrogen ions (H+).
An alkali is source of hydroxide ions (OH–).
Metal oxides, metal hydroxides and ammonia (NH₃) are called bases.
Bases neutralise acids by combining with the hydrogen ions in them.
The key reaction is:
acid + base → salt + water
An example of this is:
sulfuric acid + copper oxide → copper sulfate + water
H₂SO₄ + CuO → CuSO₄ + H₂O
Acid reactions summary
alkali + acid → water + salt
base + acid → water + salt
carbonate + acid → water + salt + carbon dioxide
metal + acid → salt + hydrogen
To assist remembering this list, many pupils find it useful to remember this horrid looking but very effective mnemonic:
AAWS
BAWS
CAWS CoD
MASH
Acids are a source of hydrogen ions (H⁺) when in solution. When the hydrogen in an acid is replaced by a metal, the compound is called a salt. The name of the salt depends on the acid used. For example if sulfuric acid is used then a sulfate salt will be formed.
| Parent acid | Formula | Salt | Formula ion |
|---|---|---|---|
| sulfuric acid | H2SO4 | sulfate | SO42- |
| hydrochloric acid | HCl | chloride | Cl- |
| nitric acid | HNO3 | nitrate | NO3- |
Acid + Alkali and Acid + Base
A base is a substance that can neutralise an acid, forming a salt and water only.
Alkalis are soluble bases. When they react with acids, a salt and water is formed. The salt formed is often as a colourless solution. Alkalis are a source of hydroxide ions (OH⁻) when in solution.
alkali + acid → water + salt
base + acid → water + salt
Examples of acid + alkali reactions:
Example of an acid + base reaction:
CuO (s) + H₂SO₄ (aq) → CuSO₄ (aq) + H₂O (l)
Acid + Carbonate
carbonate + acid → water + salt + carbon dioxide
A carbonate is a compound made up of metal ions and carbonate ions. Examples of metal carbonates are sodium carbonate, copper carbonate and magnesium carbonate.
When carbonates react with acids, bubbling is observed which is the carbon dioxide being produced. If the acid is in excess the carbonate will disappear.
Examples of acid + carbonate reactions:
Acid + Metal
metal + acid → salt + hydrogen
Metals will react with an acid if the metal is above hydrogen in the reactivity series.
When metals react with acids, bubbling is observed which is the hydrogen being produced. If the acid is in excess the metal will disappear.
Examples of acid + metal reactions:
Underneath are 3 acid reactions :
alkali + acid → water + salt
base + acid → water + salt
carbonate + acid → water + salt + carbon dioxide
A base is a substance that neutralises an acid by combining with the hydrogen ions in them to produce water.
A base usually means a metal oxide, a metal hydroxide or ammonia.
Alkalis are bases which are soluble in water.
Some metal oxides are soluble in water and react with it to form solutions of metal hydroxides. For example:
Na₂O (s) + H₂O (l) → 2NaOH (aq)
Apart from this and other group 1 oxides (such as potassium oxide) most other metal oxides are not soluble in water.
One exception is calcium oxide which does dissolve slightly in water to form calcium hydroxide (known as limewater):
CaO (s) + H₂O (l) → Ca(OH)₂ (aq)
Ammonia is another base. Ammonia reacts with water to form ammonium ions and hydroxide ions:
NH₃ (aq) + H₂O (l) ⇋ NH₄⁺ (aq) + OH⁻ (aq)
All the solutions produced here contain hydroxide ions (OH⁻) so they are all alkalis.
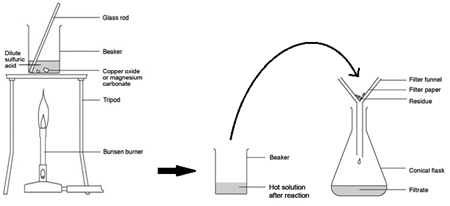
Excess Solid Method:
Preparing pure dry crystals of copper sulfate (CuSO4) from copper oxide (CuO) and sulfuric acid (H2SO4)
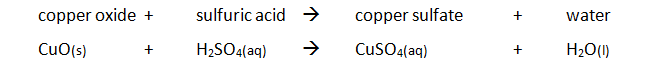
| Step | Explanation |
|---|---|
| Heat acid (H2SO4) in a beaker | Speeds up the rate of reaction |
| Add base (CuO) until in excess (no more copper oxide dissolves) and stir with glass rod | Neutralises all the acid |
| Filter the mixture using filter paper and funnel | Removes any excess copper oxide |
| Gently heat the filtered solution (CuSO4) | To evaporate some of the water |
| until crystals form on a glass rod | Shows a hot saturated solution formed |
| Allow the solution to cool so that hydrated crystals form | Copper sulfate less soluble in cold solution |
| Remove the crystals by filtration | Removes crystals |
| Dry by leaving in a warm place | Evaporates the water |
Test for hydrogen gas (H2)
Test for carbon dioxide (CO2)
If the sample is pure water it will boil at 100oC